Breakthrough in Nature Photonics: Chen Liangyi's team invented the SACD method to achieve high-throughput super-resolution imaging
Information source: Chen Liangyi researcher's team
On June 15, 2023, the team led by Professor Liangyi Chen from Peking University, in collaboration with the team of Professors Haoyu Li and Weisong Zhao from Harbin Institute of Technology, published a paper titled "Enhanced detection of fluorescence fluctuations for high-throughput super-resolution imaging"1 online in Nature Photonics. They proposed a technique that involves preprocessing the images using a pre-deconvolution method to enhance the switchable contrast of fluorescence fluctuations, thereby reducing the required number of original frames for reconstruction by at least two orders of magnitude. Additionally, they performed deconvolution again after computing the auto-correlation cumulants to further enhance the resolution of the results. Based on these principles, they introduced the Super-resolution imaging based on Auto-Correlation with two-step Deconvolution (SACD) method, which achieves the highest throughput super-resolution imaging in live cells without the need for additional hardware. The imaging requirement has been reduced from 1000 frames to just 20 frames, resulting in over a twofold improvement in three-dimensional spatial resolution. When combined with a commercial Spinning Disk Confocal (SDC) microscope, it enables rapid millimeter-scale field imaging or four-dimensional live imaging.

Super-resolution imaging, which surpasses the limitations imposed by diffraction, has become a crucial research tool in the field of life sciences. It allows for high-precision visualization and dynamic tracking of fine cellular structures and biomolecules at the nanoscale. Achieving ultra-high resolution, long-duration, and high-throughput live cell super-resolution imaging has been a primary research focus of Professor Liangyi Chen's group. In 2018, the research group introduced the highly sensitive Hessian-structured illumination super-resolution microscope (Hessian SIM), achieving a spatial resolution of 85 nanometers2. To further push the boundaries of fluorescence microscopy resolution, in 2022, the group invented the Sparse-SIM, a structure illumination microscope based on sparse deconvolution. This innovation achieved the fastest imaging speed (564 Hz) and the longest imaging duration (over 1 hour) at a spatial resolution of 60 nm3. While super-resolution microscopy can achieve remarkable resolution, it has limitations in terms of throughput. In comparison to super-resolution microscopy, confocal fluorescence microscopy is more readily available in laboratory settings. It offers optical sectioning along the z-axis and is easier to implement for high throughput, making it a preferred choice among biologists. However, its achievable spatial resolution is quite limited. Super-resolution optical fluctuation imaging (SOFI)4, based on the physical properties of fluorescence fluctuations, can be combined with confocal systems to break the diffraction limit without the need for additional optical components. However, it typically requires more than 1000 original frames to generate a super-resolution image, and its temporal resolution may not meet the demands of biomedical research for dynamic imaging of transient cellular processes.
Unlike traditional structured illumination microscopy, where imaging depth is limited, SACD technology applied to a spinning disk confocal microscope enables three-dimensional resolution extension deep within cells. Initially, researchers measured the three-dimensional resolution (Figure 1a) by randomly dispersing individual fluorescent probe particles on a substrate and observing them through the spinning disk confocal microscope. They measured the lateral/axial full width at half maximum (FWHM) of this point source to be 325/655 nanometers, which improved to 296/510 nanometers in the reconstruction results using SOFI and further improved to 135/33 nanometers in the SACD reconstruction results (all based on 20-frame reconstructions). This demonstrates that SACD can effectively achieve over a twofold resolution improvement in three dimensions.
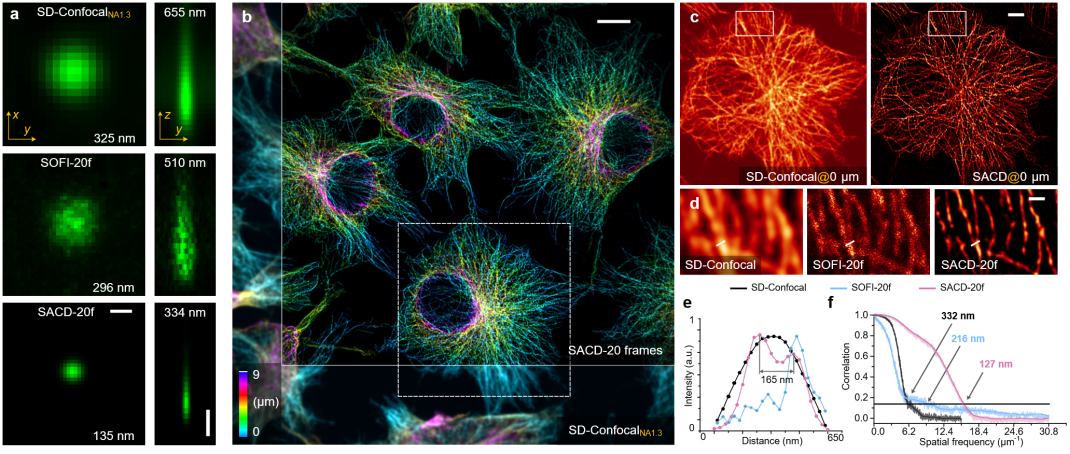
Figure 1. Three-dimensional deep super-resolution imaging with SACD. (a) Maximum intensity projection (left) and vertical cross-section (right) of single fluorescent probe particle imaging using a spinning disk confocal microscope (top), 20-frame SOFI (middle), and 20-frame SACD (bottom). (b) Pseudocolored three-dimensional image of microtubule protein in COS-7 cells, captured using a spinning disk confocal microscope (bottom left) and SACD (top right). (c) Enlarged horizontal profile of the white dashed box in (b). (d) Enlarged region of the white dashed box in (c). (e) Microtubule filament intensity distribution at the white line positions in (d), with results from the spinning disk confocal microscope (black), 20-frame SOFI (blue), and 20-frame SACD (red), with numerical values indicating distances between peaks. (f) FRC resolution analysis of the reconstructed images. Scale bars: (a, lateral and axial) 500 nanometers; (b) 10 micrometers; (c) 5 micrometers; (d) 1 micrometer.
Next, researchers demonstrated the true three-dimensional super-resolution capabilities of SACD by imaging the cellular microtubule cytoskeleton. Since SACD doesn't require specific optical illumination and configurations, it can directly employ an sCMOS camera for three-dimensional super-resolution imaging, thereby offering a larger field of view. Leveraging this advantage, researchers imaged multiple COS-7 cells simultaneously within a depth range of 9 micrometers, achieving a twofold resolution enhancement (Figure 1b). In this setup, the quality and continuity of the 20-frame SOFI images were limited (Figure 1d). In contrast, the 20-frame SACD (Figure 1d) produced high-quality reconstructed images, as shown in Figure 1e, where microtubule filaments separated by a distance of 165 nanometers were distinctly resolved. FRC resolution analysis (Figure 1f) also indicated that SACD (127 nanometers, red) exhibited significantly superior resolution compared to 20-frame SOFI (216 nanometers, blue), and this advantage was evident across all spatial frequencies.
Due to its independence from additional optical components and compatibility with fluorescence probes, SACD can directly provide super-resolution capabilities to various fluorescence imaging systems. Researchers combined the SACD algorithm with a commercial spinning disk confocal microscopy system, enabling high-throughput super-resolution imaging of microtubules within millimeter-scale fields of view (2 millimeters × 1.4 millimeters), encompassing as many as 2000 cells (Figure 2a). While traditional SOFI requires nearly half a day of continuous sampling to complete reconstructions for such large areas, SACD achieves similar or even superior imaging performance in just 10 minutes. Researchers conducted live cell imaging of two nucleolar proteins, Nop56 and B23 (Figure 2b-e), demonstrating that SACD reliably enhances resolution while maintaining linear intensity and offering higher contrast compared to SIM. In summary, SACD exhibits wide applicability across various types of biological samples.
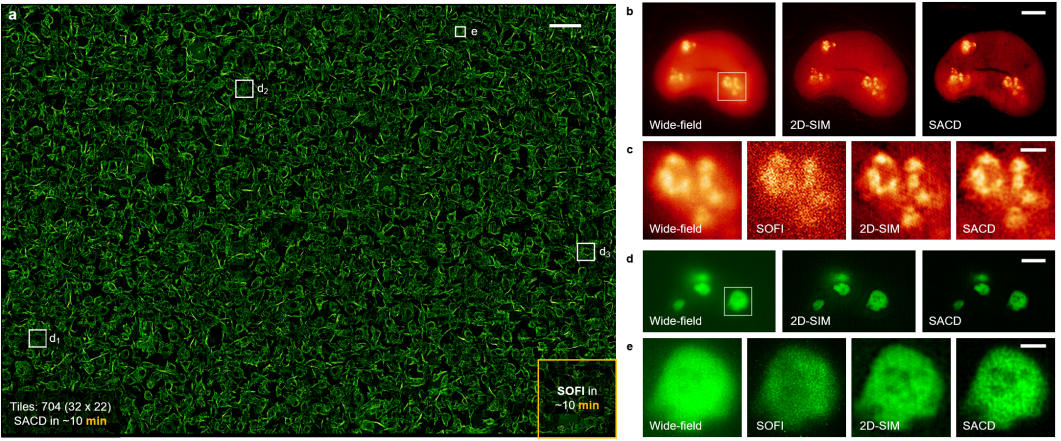
Figure 2. High-throughput Super-resolution Imaging with SACD. (a) High-throughput super-resolution imaging achieved by SACD (20 frames) within a 2.0 millimeter × 1.4 millimeter area containing over 2000 cells, depicting the microtubule protein imaging in COS-7 cells. (b) Imaging results of live COS-7 cells labeled with Nop56-Skylan-S on wide-field microscopy (left), 2D-SIM (middle), and SACD (right). (c) Comparative results (within the white box shown in (b)) from wide-field microscopy (1st column), SOFI (2nd column, 20 frames), 2D-SIM (3rd column), and SACD (4th column). (d) Imaging results of live COS-7 cells labeled with B23-rsFusionRed3 on wide-field microscopy (left), 2D-SIM (middle), and SACD (right). (e) Comparative results (within the white box outlined in (d)) from wide-field microscopy (1st column), SOFI (2nd column, 20 frames), 2D-SIM (3rd column), and SACD (4th column). Scale bars: (a) 100 micrometers; (b, d) 5 micrometers; (c, e) 1 micrometer.
movie1: High throughput imaging - large field of view cytoskeletal microtubule imaging
Achieving truly high stability and fidelity in live cell super-resolution imaging has always been challenging, primarily due to severe limitations posed by signal-to-noise ratio, photobleaching, and phototoxicity. In this context, researchers introduced a previously developed sparse deconvolution method3, primarily employing spatiotemporal continuity and sparsity to perform background pre-filtering and subsequent deconvolution on the original images, maximizing the utilization of information within the raw images (Sparse-SACD). Researchers employed SACD technology for comprehensive four-dimensional super-resolution imaging of live cells, specifically three-dimensional super-resolution imaging of the mitochondrial outer membrane network in COS-7 live cells over a period of more than 10 minutes (imaged at 37°C) (Figure 3). Compared to traditional confocal systems, the visibility of fine structures was significantly enhanced, and mitochondria were imaged as sharp, hollow membrane structures. Through the dual constraints of sparsity and continuity, SACD faithfully achieved real-time whole-cell imaging of mitochondrial fission and fusion.
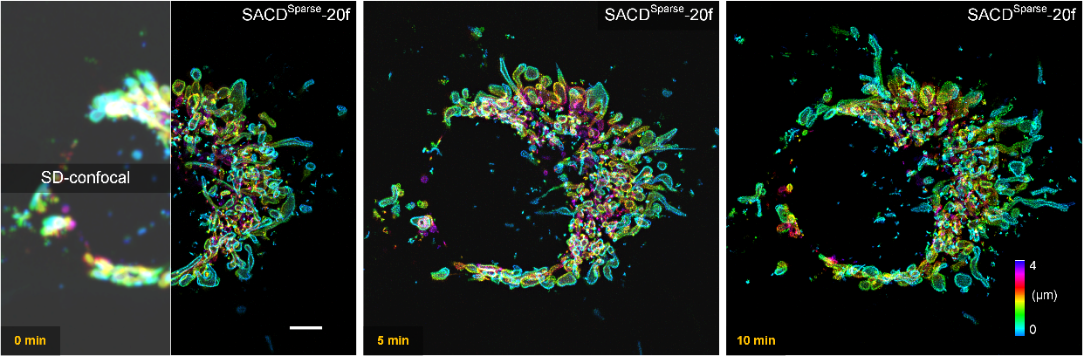
Figure 3. Live Four-Dimensional Super-Resolution Imaging with SACD. Four-dimensional imaging of live COS-7 cells labeled with Skylan-S-TOM20 was performed using a spinning disk confocal microscope (SD-confocal, left) and Sparse-SACD (right) at 37°C. Scale bar: 5 micrometers.
Movie2. MP4 Comparison of continuous imaging results of confocal, SOFI, SACD, Sparse-SACD in living cell skeleton (MAP4)
By harnessing the fluorescence fluctuations present in the raw images, SACD can directly enhance three-dimensional resolution on commercial systems. Consequently, researchers anticipate SACD to become a routine tool for biologists to analyze complex transient dynamics within live cells, enabling high-throughput, high-resolution, and cost-effective microscope-based screening.
Assistant Professor Wei-Song Zhao from Harbin Institute of Technology, Associate Researcher Shi-Qun Zhao from Peking University, and doctoral students Zhen-Qian Han and Xiang-Yan Ding, all share joint first authorship on this paper. Professor Hao-Yu Li from Harbin Institute of Technology and Professor Liang-Yi Chen from the National Biomedical Imaging Center at Peking University are the corresponding authors of the paper. Additionally, co-corresponding authors include Professor Xu-Min Ding from Harbin Institute of Technology and Associate Researcher Chang-Liang Guo from the National Engineering Research Center for Beijing/Tianjin/Hebei Technology Innovation at Peking University. The data processing for this project received hardware and technical support from Peking University's "Weiming" Supercomputing Platform. This research was supported by the National Natural Science Foundation of China, the National Key R&D Program, the Beijing Natural Science Foundation, and the Joint Life Science Center, among others.
The article link:https://doi.org/10.1038/s41566-023-01234-9
References:
1. Zhao W, Zhao S, Han Z, et al. Enhanced detection of fluorescence fluctuations for high-throughput super-resolution imaging. Nature Photonics, 2023: DOI: https://doi.org/10.1038/s41566-023-01234-9.
2. Huang X, Fan J, Li L, et al. Fast, long-term, super-resolution imaging with Hessian structured illumination microscopy. Nature Biotechnology, 2018, 36(5): 451-459.
3. Zhao W, Zhao S, Li L, et al. Sparse deconvolution improves the resolution of live-cell super-resolution fluorescence microscopy. Nature Biotechnology, 2022, 40(4): 606-617.
4. Dertinger T, Colyer R, Iyer G, et al. Fast, background-free, 3D super-resolution optical fluctuation imaging (SOFI). PNAS, 2009, 106(52): 22287-22292.




