Imaging microglia surveillance during sleep-wake cycles in freely behaving mice
Information source: Professor Cheng Heping's team
Sleep is an indispensable part of our lives, but the regulatory mechanisms behind it are still very mysterious. Recently, a study by a collaborative team of Cheng Heping from Peking University and Gu Xiaochun from Southeast University reported the important role of microglia in natural sleep, shedding light on the corner of the sleep mystery.
Microglia are intrinsic immune cells of the central nervous system, accounting for 10-15% of the entire brain cells. They play key roles in neurogenesis, synaptic pruning, damage repair, and learning and memory, yet their role in natural sleep has been unclear. Microglia are very sensitive to the brain microenvironment, and the inflammatory response triggered by traditional invasive craniotomy alters the physiological morphology of the cells, which somewhat limits the study of microglia function in natural sleep. In order to study and solve these problems, the team adopted a non-invasive cranial window embedding method and used miniaturized two-photon microscopic imaging to successfully acquire microglia dynamic contact images of freely behaving mice for 10 consecutive hours, and combined with EEG/EMG recordings and behavioral videos (Figure 1) to investigate the morphodynamics of microglia during the natural sleep-wake cycle and their regulatory mechanisms. The tiny size of the miniaturized two-photon microscope makes it possible to wear it on the animal's head without interfering with its sleep state; the low photobleaching property of two-photon imaging makes it possible to carry out the whole imaging process throughout the entire sleep cycle of the mice without affecting the fluorescence quality, which allows us to have a glimpse of the dynamic changes of microglia during the sleep-wake cycle.
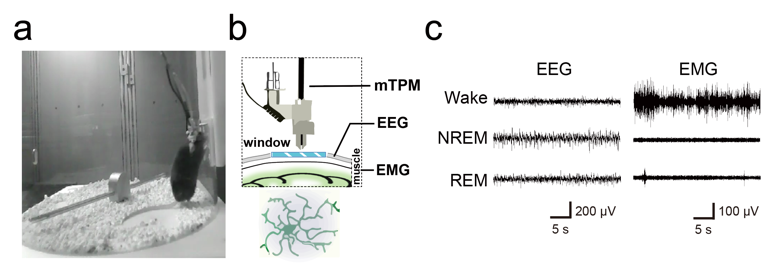
Fig. 1: Freely behaving mice wearing miniaturized two-photon probes and cerebral EMG devices on their heads
The study found that microglia morphology has its own typical dynamics in wakefulness, non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep (Figure 2), and that 3 hours of sleep deprivation significantly altered microglia morphology. Subsequently, the team used a norepinephrine (NE) probe to image NE dynamics in the cerebral cortex in real time, and further explored the regulatory mechanisms of microglia morphology in conjunction with intracranial drug delivery. It was further found that NE levels in the cerebral cortex play a key role in the regulation of microglia morphological changes. Oscillations in NE levels in the cortex occur in a sleep state-dependent manner, and β2-adrenergic receptor signaling plays an important role in microglia morphodynamic changes. This provides new insights into the mysterious mechanism of sleep and a new paradigm for future research to find the "dark matter" that drives sleep. The results were published in the internationally recognized journal eLife under the title of "Imaging microglia surveillance during sleep-wake cycles in freely behaving mice". Xiaochun Gu, Associate Professor of Southeast University, and Chong Zhao, PhD student of Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, are the co-first authors of the article, and Xiaochun Gu and Academician Heping Cheng are the co-corresponding authors of the article.
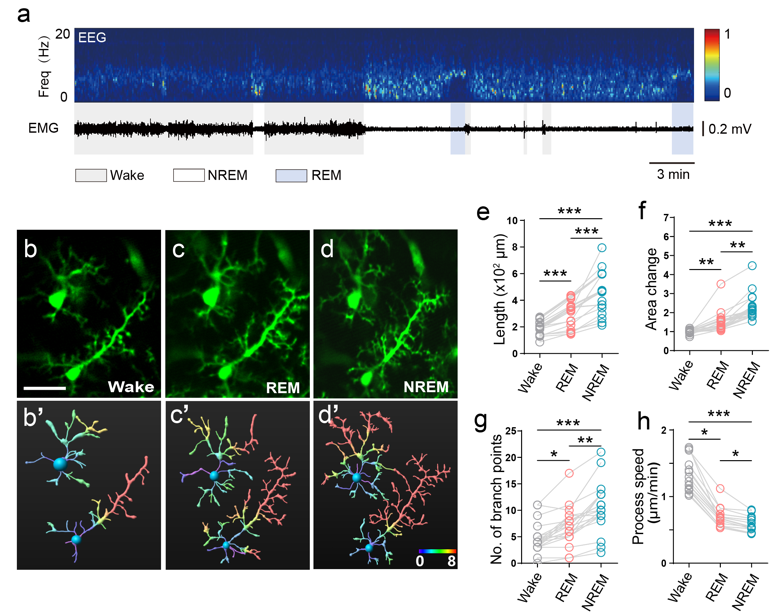
Fig. 2: Morphological differences in microglia in different brain states
The weight of the miniaturized two-photon probe is only 2.2g, which can meet the demand of free behavioral imaging in mice for 24 consecutive hours, and opens up a new research pathway for future studies on the physiological functions of microglia in natural sleep. This technology enables us to explore the microcosm of the brain more deeply, and we look forward to future studies that will reveal more secrets between microglia and sleep, and provide new possibilities for improving people's sleep quality.
eLife assessment: “the article is an exceptional technical feat to bridge a crucial gap in understanding sleep state-induced dynamics of microglia and its modulation by norepinephrine signaling”.
Original link: https://doi.org/10.7554/eLife.86749.3




