Liangyi Chen's team develops readily releasable β cells with tight Ca2+–exocytosis coupling dictate biphasic glucose-stimulated insulin secretion
Information source: Professor Chen Liangyi’s team
On January 26, 2024, Liangyi Chen's team at Peking University published the paper "Releasable β cells with tight Ca2+-exocytosis coupling dictate biphasic glucose- stimulated insulin secretion" online in Nature Metabolism. By developing high temporal and spatial resolution fluorescence imaging of pancreatic islet tissue and computer-assisted image processing analysis algorithms to study intact and undamaged pancreatic islets across scales, they found that there is only a small population of β-cells in the islets that secrete insulin, called Readily releasable b cells (RRβs). RRβs are functional β-cells in the pancreatic islets and dominate the glucose-stimulated biphasic secretion of insulin. RRβs form the first phase of insulin by synchronized secretion and the second phase by continuous secretion, which is the central regulatory mechanism at the level of pancreatic islet organization. Dysfunctions of RRβs are closely related to the development of diabetes mellitus.

Biphasic glucose-stimulated insulin secretion (GSIS) is a condition that can stimulate rapid transient first-phase secretion of insulin and slow sustained second-phase secretion of insulin when blood glucose rises sharply. It was discovered in the late 1960s that reduced insulin first-phase secretion is an early marker of impaired beta-cell function (PMID:11484070). In the first decade of this century, electrophysiology (diaphragm technology), molecular biology and Total Internal Reflection Fluorescence microscopy (TIRFM) were utilized to propose the existence of β-cells based on multiple dimensions of membrane capacitance, vesicle spatial location in single cells, as well as molecular knockout experiments at the whole animal level. It was proposed that there are different insulin vesicle pools in β-cells involved in fast and slow insulin secretion, respectively (PMID:11390882). This is the currently recognized mechanism of GSIS biphasic secretion. However, this model does not take into account the functional differences of different β-cells at the islet level (PMID:29559510) as well as the regulatory role of other islet endocrine cells (e.g., α and δ cells) (PMID:16461897). Thus, how insulin secretion is regulated at the islet level remains unclear. And the biggest challenge in exploring this question is the lack of technical means to detect insulin secretion from different β-cells within pancreatic islet tissues.
Prof. Liangyi Chen's group has long been engaged in the development of high temporal and spatial resolution fluorescence imaging technology to explore the mechanism of abnormal insulin secretion in the process of diabetes. Using the characteristic of co-release of Zn2+ and insulin, the authors used zinc ion fluorescent dye to selectively label insulin fusion vesicles, and combined with high-speed turntable confocal microscope, they invented "zinc scintillation" which can detect the secretion of insulin vesicles from different β-cells in the pancreatic islet at the same time. Using the "zinc scintillation" technique, the authors observed hundreds or thousands of insulin vesicle secretion events in a single pancreatic islet, quantitatively analyzed the spatial and temporal distribution of these secretion events, and found that only a small fraction of pancreatic β-cells secreted insulin and most of the β-cells seldom secreted insulin in response to physiological glucose stimulation (Figure 1).
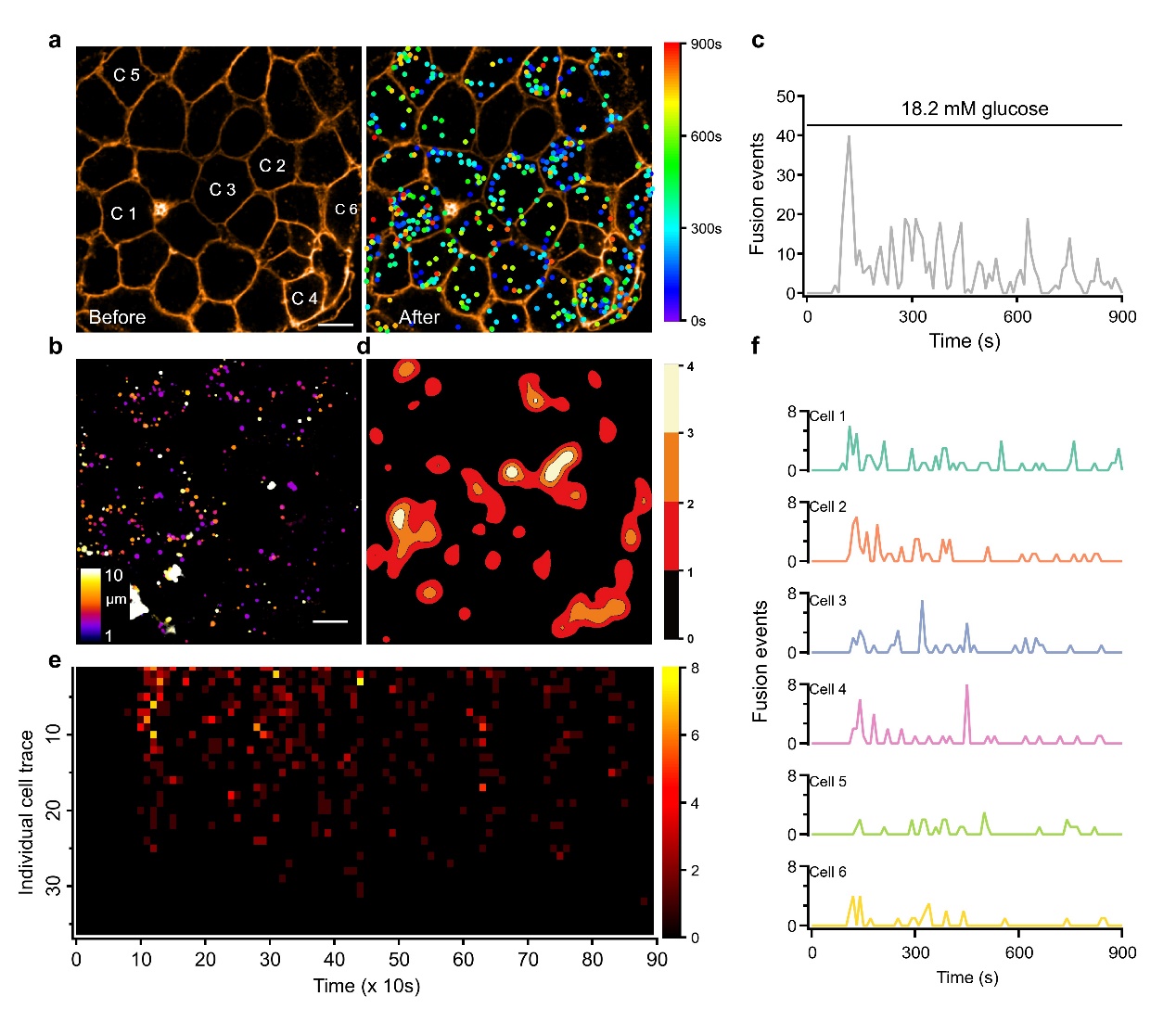
Fig. 1. Heterogeneous insulin secretion in glucose-stimulated pancreatic islets. a, Spatiotemporal distribution of insulin vesicle secretion events before and after 18.2 mM glucose stimulation. The position of the dots shows where the secretion occurred, and the color represents the time. b, Distribution of secretion events in three-dimensional space. c, Dynamic secretion process of insulin vesicles. d, Contour density map of the secretion events. e, Secretion process of a single pancreatic islet cell. One row represents one cell and one pixel represents the number of vesicles secreted in 10 s. f, Detailed maps of the secretion dynamics of the six cells selected in Figure a. The scale bar is 10 μm. Scale bar is 10 μm.
Through a series of mathematical tests, they found an exponential distribution of the secretory capacity of beta cells within the pancreatic islets. Quantitative assessment of this heterogeneity using the Gini coefficient indicated that approximately 40% of the islet cells contribute 80% of the secretory events of the entire islet. Therefore, this group of more secretory b-cells was defined as RRβs, and the remaining β-cells that do not secrete insulin in response to glucose stimulation were defined as Releasable-incompetent β cells (RIβs). Repeating two rounds of the same glucose stimulation to the same islet resulted in an 83% overlap of RRβs, demonstrating that they are functional β cells that are stably present in pancreatic islets. The two-phase secretion of insulin was determined by RRβs, whose synchronized secretion formed a fast first phase, while asynchronous secretion produced a slow second phase. This suggests that pancreatic islet β-cells emerge from the characteristics of biphasic secretion through some collective behavior that goes beyond their basic constituent units.
Using Ins1-GCaMP6f mice, a mouse model in which b-cells specifically express Ca2+ indicators, and a novel high-performance zinc probe, PKZnR-1 (PMID: 34423531, synthesized by Zhixing Chen's team at Peking University), the authors demonstrated that despite the several-fold difference in secretion capacity between RRβs and RIβs, there is no significant difference in Ca2+ signaling. This is in contrast to recently reported "high-functioning" b-cell subpopulations categorized based on Ca2+ signaling characteristics, such as Hub/Leadercells (PMID: 27452146; PMID: 32694805), 1st responder cells (PMID: 36099294), etc. The authors demonstrated that RRβs and RIβs do not differ significantly in Ca2+ signaling despite the several-fold difference in secretion capacity, which also suggests that Ca2+ signaling does not refer to islet β-cell function. The functional differences between RRβs and RIβs are mainly affected by the efficiency of Ca2+-secretion coupling, which is a result of the combined action of neighboring a-cells and d-cells.
Finally, the authors explored the role of RRβs cells in the development of diabetes. The number of RRβs was significantly reduced on pancreatic islets of leptin-deficient ob/ob mice (50% vs. 30% at 4 weeks of age and 42% vs. 27% at 8 weeks of age, Figure 2a-2b). Moreover, b-cell Ca2+ signaling in ob/ob mice shifted from Constant mode to Spike mode in normal mice, resulting in a "Burst" of RRβs to secrete insulin (Figure 2c-2d). These phenomena suggest that RRβs are the core of insulin secretion regulation at the islet level, and that dysfunction of RRβs is a key component of islet dysfunction.
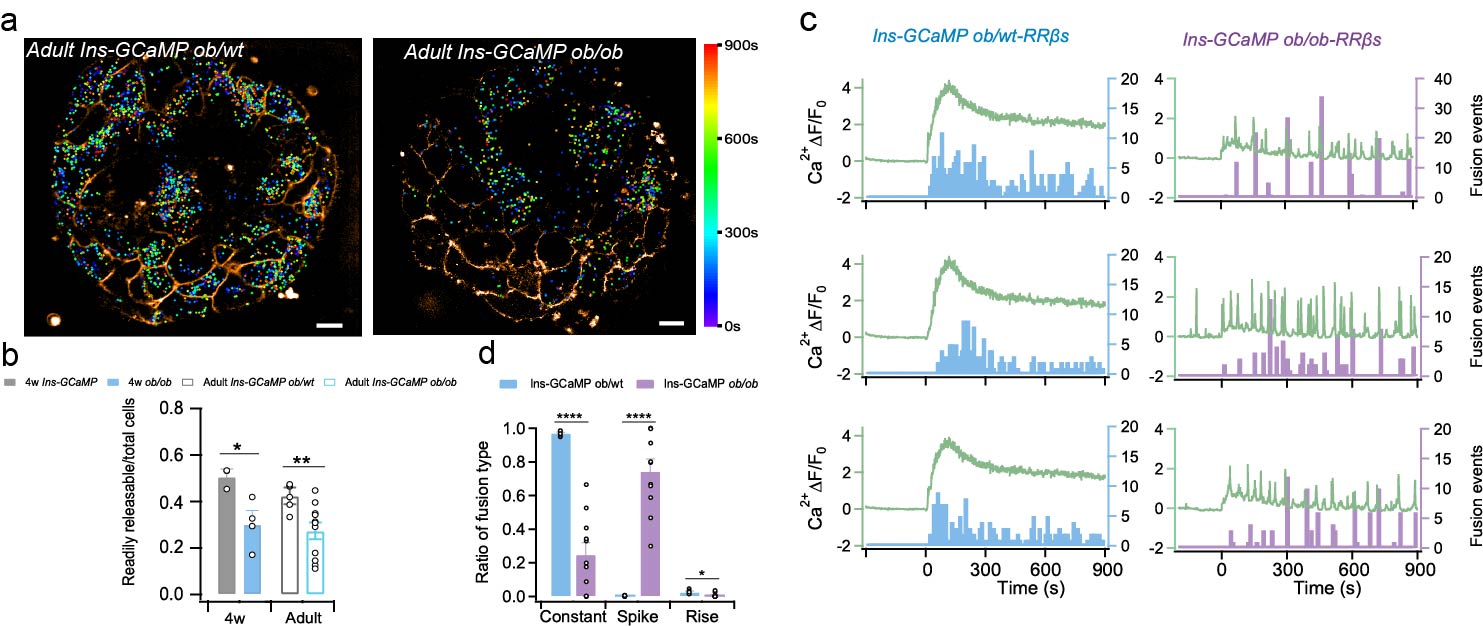
Fig. 2. Abnormal insulin secretion in pancreatic islets of ob/ob mice. a, Spatiotemporal distribution of insulin vesicle secretion in pancreatic islets of adult ob/ob mice and their littermate heterozygous littermates. b, Proportion of RRβs cells in pancreatic islet cells. c, Calcium signals (curves) versus insulin vesicle secretion events (bars) in RRβs cells in pancreatic islets of ob/ob mice and their littermate heterozygous littermates. d, Different calcium signaling-triggered secretion event ratios.
Overall, this study identifies functional β-cells, RRβs cells, in pancreatic islets by cross-scale islet tissue imaging, thus answering a mechanism that has been unclear for five decades: it is RRβs cells, not the vesicle pool in each islet b-cell, that determine glucose-stimulated biphasic insulin secretion. This finding also suggests that the organization of individual b cells into a complete pancreatic islet is functionally limited on an individual basis, thus emerging as a physicochemical property and regulatory mechanism inherent to the level of pancreatic islet organization. Such regulatory mechanisms cannot be fully elucidated by cellular-level, molecularly manipulated experiments. Therefore, combining studies at different scales and developing high-resolution functional imaging methods at different scales are often the way to find key mechanisms in physiology and pathology.
The work was published online at the same time that Nature Metabolism featured it in a Research Briefing (https://www.nature.com/articles/s42255-023-00964-y). The group also published the authors' 13-year story "From a new method to a new mechanism: the Long March to understand how islet insulin secretion is regulated by glucose," in Nature research communities (https://communities.springernature.com/posts/from-a-new-method-to-a-new-mechanism-the-long-march-to-understand-how-islet-insulin-secretion-is-regulated-by-glucose-stimulation).
Prof. Liangyi Chen of Peking University and Researcher Huisheng Liu of Guangzhou National Laboratory are the co-corresponding authors of this paper. Xiaohong Peng (former postdoctoral fellow, now supervising technician), Huixia Ren (postdoctoral fellow), Lu Yang (assistant researcher), and Shiyan Tong (graduated PhD student) of Peking University are the co-first authors of this paper. Academician Chao Tang of Peking University, Academician Tao Xu of Guangzhou National Laboratory, Prof. Zhixing Chen of Peking University, and Prof. Yongdeng Zhang of Westlake University provided significant support and assistance for this study. This study was supported by the New Cornerstone Science Foundation, the National Outstanding Youth Foundation and the National Key Research and Development Program Fund.
Original link: https://www.nature.com/articles/s42255-023-00962-0




