Liangyi Chen's team develops super-resolution scale in situ imaging quality assessment tool
Information source: Professor Chen Liangyi's team
On December 14, 2023, Liangyi Chen's team at Peking University and Weisong Zhao/Haoyu Li's team at Harbin Institute of Technology collaborated to publish the paper Quantitatively mapping local quality of super- resolution microscopy by rolling Fourier ring correlation in Light: Science & Applications (IF: 20.3). They proposed that the rolling Fourier ring correlation (rFRC) method can be utilized to evaluate the reconstruction uncertainty at super-resolution scales. Meanwhile, by combining the rFRC method with the improved Resolution scaled error map (RSM), a Pixel-level analysis of error locations (PANEL) is proposed, which can generate the quantitative pixel-level error map at super-resolution scale in a general and reference-free way. PANEL pinpoints the less reliable regions for bioanalysis, and improves the determination accuracy by up to 10 times. In addition, rFRC can accurately compare the performance of different Single-molecule localization microscopy (SMLM) recovery algorithms, which facilitates the effective fusion of different reconstructed images at the super-resolution scale, minimizes potential errors, and achieves nanoscale extreme spatial resolution imaging of single molecule localization in a large field of view. The team provided rFRC with complete MATLAB and Python open source code, as well as out-of-the-box Fiji/ImageJ plug-ins, which enable it to be combined with a variety of modal optics (super-resolution) imaging techniques, making it an easy-to-use local quality assessment tool.

By setting up fluorescent probes or incorporating physical phenomena of excited radiation, super-resolution fluorescence microscopy has broken through the physical diffraction limit of resolution (200 to 300 nanometers). However, the ability of super-resolution microscopy to extract super-resolution information from samples often relies on the computational reconstruction and subsequent processing of the image, and factors such as the chemical environment and the optical setup can have an impact on the image in the reconstruction, resulting in noise and distortion, and degrading the quality of the super-resolution image. Therefore, accurate and reliable quality assessment of super-resolution images can effectively quantify errors and uncertainties to further analyze life science issues. Although there are several methods to assess the quality of super-resolution images, it is not yet possible to perform ultra-precise and reference-free quantitative analysis at the super-resolution scale, and it is difficult to accurately evaluate the heterogeneity of image resolution distribution.
In order to solve the above problems, Liangyi Chen's team uses rolling Fourier loop correlation computation to quantize and estimate the pixel-level small errors of the image on the super-resolution scale. At the same time, for larger structural distortions and other errors, an improved resolution scale error map (RSM) is introduced, which ultimately constitutes a complete set of super-resolution scale microscopic image reconstruction quality assessment program (Pixel-level ANalysis of Error Locations-PANEL). Using this technique, the performance of different single-molecule localization microscopy reconstruction algorithms can be accurately compared, and the effective fusion of different reconstructed images at the super-resolution scale can be further facilitated to minimize the potential errors. In addition, the method can be combined with multiple modal optical super-resolution microimaging techniques commonly used today, making it an easy-to-use analytical tool for image localization quality assessment.
The researchers validated the proposed assessment method using a single-molecule localized microtubule dataset (Fig. 1). Super-resolved microtubule 2D images obtained by large-field-of-view STORM showed that the resolution of filament crossings and the perinuclear region of the cell, due to projectile aggregation and defocusing effects, was significantly lower (Fig. 1 left). The cytoskeleton exhibits a gradual thinning from the perinuclear region to the peripheral region from the uncertainty quantitative evaluation and resolution distribution maps given in the figure, demonstrating that the method successfully maps the resolution heterogeneity induced by changes in microtubule densities (Fig. 1 right). The single-molecule localization microscopy (STORM) resolution heterogeneity can be effectively evaluated using this method.
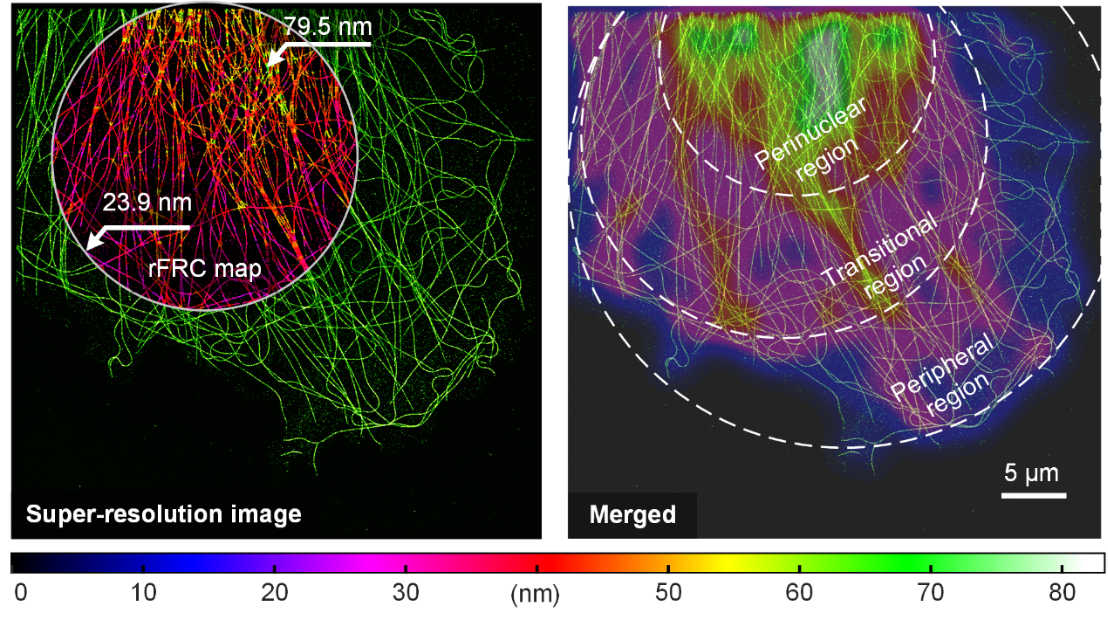
Fig. 1 | rFRC assessment of super-resolution images from localization microscopy. Left: STORM reconstruction results of α-microtubulin labeled with Alexa Fluor 647 in COS-7 cells and its rFRC plot. Right: Merged view of STORM results (bright green) and Gaussian-averaged rFRC plots (shifted jet plots) for highlighting low-quality regions. Scale bar: 5 μm
PANEL can be widely used as a super-resolution technique for evaluating other modalities due to the generality of its principle. The research team applied the Hessian denoising algorithm to the Wiener-SIM reconstruction (Fig. 2a) to obtain Hessian-SIM images (Fig. 2b). The rFRC value accurately reflects the slight difference in fidelity between Wiener-SIM and Hessian-SIM (rFRC value: 1.36 vs. 1.24) compared to the RSM values of the two images being equal (RSM value: 0.27 vs. 0.27) (Fig. 2c). In addition, rFRC can be used to determine the number of iterations of the Richardson-Lucy (RL) deconvolution algorithm. The researcher applied RL deconvolution to process the TIRF images (Fig. 2d) and calculated the corresponding rFRC values for each iteration (Figs. 2e, 2g). Analyzing the change characteristics of rFRC with the number of iterations, the number of RL deconvolution iterative reconstruction can be determined to obtain the best reconstruction results.
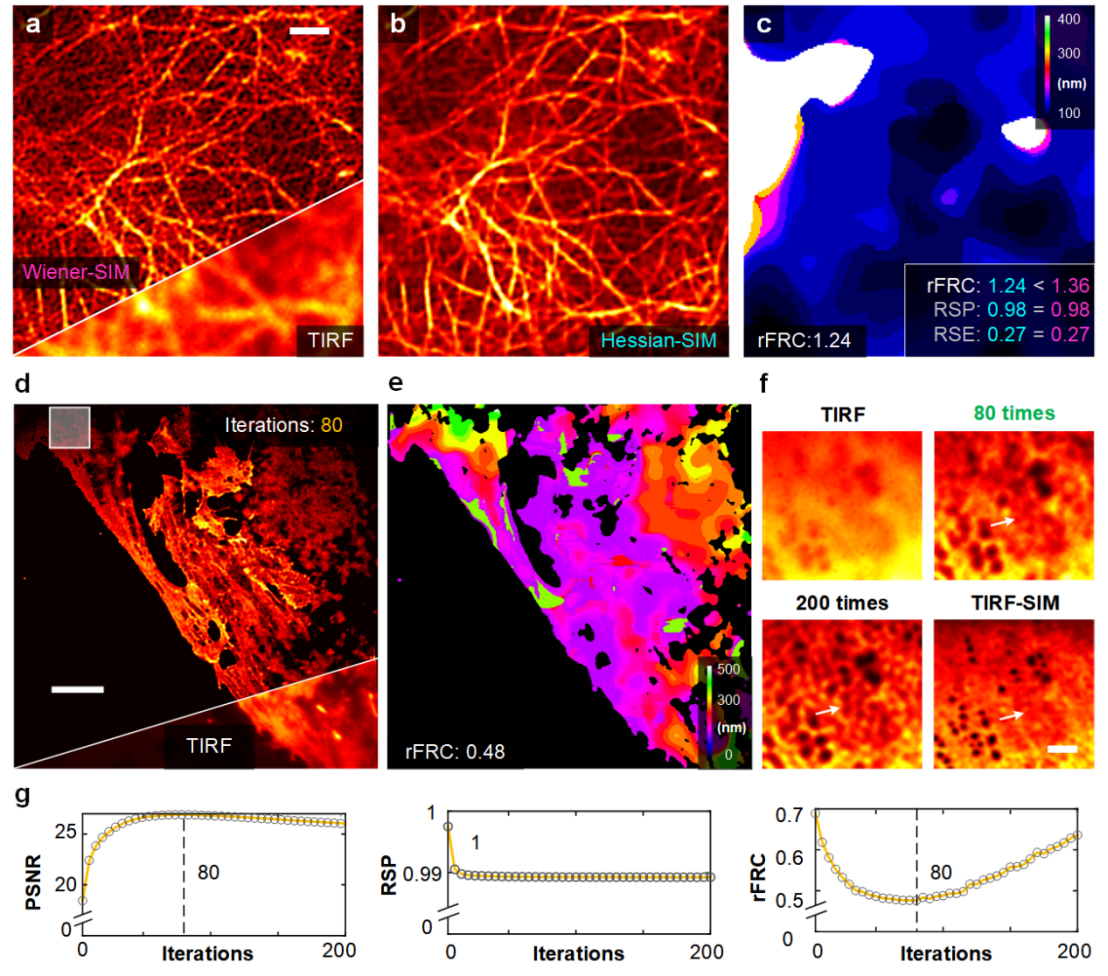
Figure 2 | rFRC maps assisting and evaluating multiple optical imaging methods. (a) Representative images of live human umbilical vein endothelial cells (HUVEC) labeled with LifeAct-EGFP under Wiener-SIM (top) and TIRF (bottom) imaging. (b) Hessian-SIM results. (c) rFRC plots for Hessian-SIM. rFRC, RSP and RSE values for Wiener-SIM (magenta) and Hessian-SIM (cyan) are shown in the lower right corner. (d) Representative results of fixed liver sinusoidal endothelial cells (LSEC) labeled with DiI under RL deconvolution (top) and TIRF (bottom) imaging. (e) rFRC plot of RL deconvolution results. (f) Magnified view of the white box in (d). The original TIRF image, the RL deconvolution results for 80 and 200 iterations, and the TIRF-SIM results are shown in the upper left, upper right, lower left, and lower right, respectively. (g) Curves of PSNR (with respect to TIRF-SIM), RSP (with respect to TIRF) and rFRC values during iterations. Scale bars: (a) 1 μm; (d) 5 μm; (f) 100 nm
Overall, rFRC and PANEL techniques reveal the uncertainty of image spatial information, which not only provides important support for biological analysis based on super-resolution images, but also has the potential to promote further development in the field of computational microscopy imaging. The researchers expect that PANEL can be widely used as a cross-modal tool and become a widely used bioanalytical method, providing a powerful tool for the innovation of super-resolution microscopy, and pushing the field of computational microscopy to achieve greater progress. Liangyi Chen's team has already developed various computational super-resolution imaging techniques (Nature biotechnology, 2022; Nature Photonics, 2023), and PANEL will support the existing computational super-resolution imaging techniques to form a truly quantitative and reproducible.
Assistant Professor Weisong Zhao of Harbin Institute of Technology (HIT) is the first author of the paper, and Professor Haoyu Li of HIT and Professor Liangyi Chen of the National Center for Biomedical Imaging Science at Peking University (NCBI) are the corresponding authors of the paper. Co-first authors of the paper include Assistant Professor Huang Xiaoshuai of Peking University and Postdoctoral Fellow Yang Jianyu of Nankai University, while co-corresponding authors include Professor Pan Leiting of Nankai University and Distinguished Associate Researcher Zhao Shiqun of Peking University. Academician Tan Jiubin of Harbin Institute of Technology is a co-author of the paper.
Original link: https://doi.org/10.1038/s41377-023-01321-0




