Chen Lei's research team found that the R-helix on SUR2A inhibits the activation of KATP channels
Information source: Chen Lei researcher's team
On June 17, 2023, the research team led by Dr. Lei Chen from the Molecular Medicine Institute of the Future Technology Institute at Peking University, the Peking University-Tsinghua Joint Center for Life Sciences, and the National Biomedical Imaging Center published a paper titled "The inhibition mechanism of the SUR2A-containing KATP channel by a regulatory helix" in the journal Nature Communications. In this study, a series of cryo-electron microscopy structures of SUR2A and SUR2B in the presence of the inhibitor repaglinide (RPG) and different Mg-nucleotides were analyzed. The researchers discovered a previously unreported helix on SUR2A that can inhibit the KATP channel. For more information, please refer to: https://www.nature.com/articles/s41467-023-39379-4.
The activity of ATP-sensitive potassium channels (KATP) is inhibited by intracellular ATP and activated by Mg-ADP. These channels couple the cellular energy metabolism level with cellular electrical signals1. KATP channels are hetero-octamers composed of four Kir6 subunits and four SUR subunits. The Kir6 subunits form the central pore region for potassium ion efflux2, while the SUR subunits, acting as regulatory subunits, modulate channel gating by binding activators (Mg-ADP, KATP openers) and inhibitors (insulin secretagogues)3. There are three subtypes of SUR subunits, SUR1, SUR2A, and SUR2B, with SUR2A and SUR2B being splice variants encoded by the ABCC9 gene, differing only in their C-terminal 42 amino acids1. SUR2A plays a crucial role in cardiac and skeletal muscle, while SUR2B is primarily found in smooth muscle2,4. Mutations in the ABCC9 gene are associated with diseases such as dilated cardiomyopathy5, familial atrial fibrillation6, and Cantu syndrome7. The SUR proteins belong to the ABC superfamily and contain two transmembrane domains (TMD) and two intracellular nucleotide-binding domains (NBD)3. NBD dimerization promotes the proximity of TMD1 and TMD28. Despite the high sequence similarity among different SUR subtypes, Mg-ADP and KATP openers have varying activation effects on different subtypes: SUR2A exhibits a weaker response to Mg-ADP activation compared to SUR1 and SUR2B9-17. This characteristic of SUR2A ensures that in normal cardiac function, it does not lead to the activation of KATP channels, thus avoiding interference with normal cardiac electrical signals. Dr. Lei Chen's research group had previously elucidated the structures of SUR2A and SUR2B in the occluded (OD) state, with NBD1 binding Mg-ATP and NBD2 binding Mg-ADP (SUR2OD/ATP/ADP)18. However, these structures did not explain why SUR2A exhibits a weaker response to Mg-ADP activation compared to other SUR subtypes.
In this research study, Dr. Lei Chen's team used RPG to inhibit the complete transition of SUR from the Inward-facing (IF) conformation to the OD conformation, and they elucidated a series of structures of SUR2A and SUR2B in the presence of Mg-nucleotides. In the structure of SUR2A binding Mg-ATP in both NBD1 and NBD2 (SUR2AIF/MgATP/MgATP), the researchers observed a distinct helical density (924-942). This helix is connected to NBD1 and bridges between NBD1 and NBD2, thus it was named the regulatory helix (R-helix). On one side, the R-helix interacts with a hydrophobic pocket composed of D789, L792, L793, and I806 on NBD1 through R930 and L933. On the other side, it interacts with ATP bound to NBD2 through Y938, K931, and R935. However, in the structure of SUR2A binding Mg-ADP in NBD2 (SUR2AIF/MgATP/MgADP), the researchers observed a certain degree of NBD dimerization, and the R-helix was not detected. This suggests that Mg-ADP disrupts the interaction between the R-helix and NBD (Figure 1).
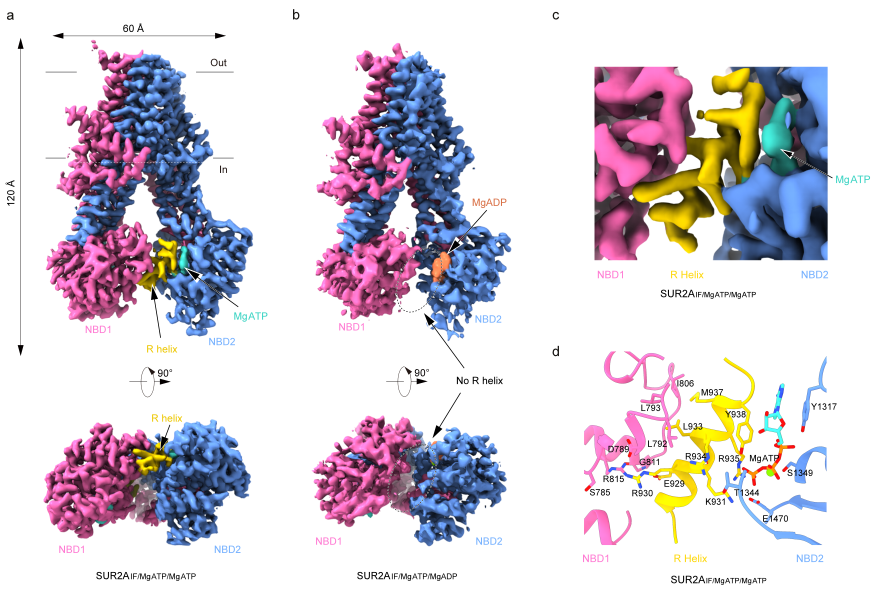
Figure 1: Electron density of R helix in SUR2A under different nucleotide binding states
In the structure of SUR2B binding Mg-ATP, researchers similarly observed the density of the R-helix, although it appeared less well-defined, suggesting its higher dynamic nature. When SUR2B bound Mg-ADP in NBD2, it predominantly exhibited two distinct conformations. In one state, similar to SUR2AIF/MgATP/MgADP, denoted as SUR2BIF/MgATP/MgADP, the density of the R-helix was blurry and discontinuous. In the other state, NBDs in SUR2BIF/MgATP/MgADP were in closer proximity, and the R-helix density was completely absent (Figure 2).
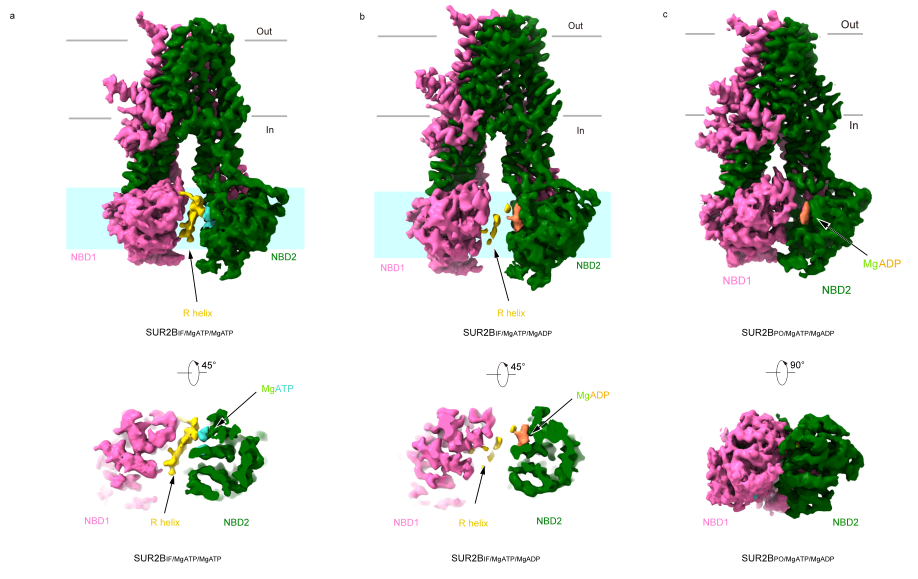
Figure 2: Conformation of SUR2B and electron density of R-helix in different nucleotide binding states
Researchers conducted electrophysiological experiments with mutant variants and discovered that mutations in the amino acids of SUR2A's R-helix that interact with NBD1 and NBD2 led to an enhanced activation of the KATP channel by Mg-ADP. This finding indicates that the R-helix plays an inhibitory role in the KATP channel, consistent with its unique spatial position.
In summary, this study elucidates the structural mechanism by which the R-helix inhibits the KATP channel by restraining the NBD dimerization of SUR2A (Figure 3). It proposes that SUR2B-C42 may promote the binding of Mg-ADP to NBD2 through allosteric effects, further leading to the dissociation of the R-helix, NBD dimerization, and the activation of the KATP channel. This work lays a foundation for further in-depth investigations into the regulatory mechanisms of different subtypes of KATP channels.
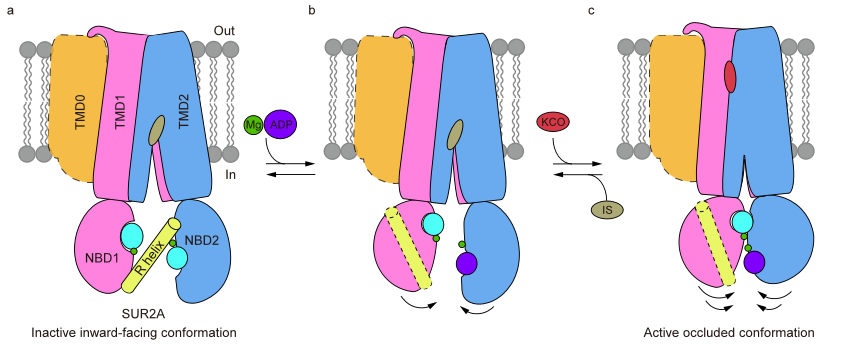
Figure 3: Structural model of R helix involved in regulating the conformational change of SUR2A protein
Ding Dian and Hou Tianyi, both doctoral students at the Institute of Advanced Interdisciplinary Studies at Peking University and the Peking University-Tsinghua University Joint Center for Life Sciences, are the co-first authors of this work. Assistance in data collection was provided by Wu Jingxiang, a postdoctoral researcher at the Institute of Molecular Medicine at the School of Future Technology of Peking University, and Wei Miao, a doctoral student. Dr. Lei Chen is the corresponding author of the paper. The preparation, screening, and collection of cryo-electron microscopy samples for this research were carried out at Peking University's Cryo-EM Platform and Electron Microscopy Facility, with significant assistance from individuals such as Xue-Mei Li, Zhen-Xi Guo, Chang-Dong Qin, Xia Pei, Xiao-Juan Hui, and Guo-Peng Wang. Data processing was supported by the hardware and technical expertise of Peking University's CLS Computing Platform and Unicore Supercomputing Platform. This project received financial support from the National Natural Science Foundation of China, the Joint Center for Life Sciences, and the State Key Laboratory of Membrane Biology.
References:
1. Nichols, C. G. KATP channels as molecular sensors of cellular metabolism. Nature 440, 470-476, doi:10.1038/nature04711 (2006).
2. Hibino, H. et al. Inwardly Rectifying Potassium Channels: Their Structure, Function, and Physiological Roles. Physiological Reviews 90, 291-366, doi:10.1152/physrev.00021.2009 (2010).
3. Vedovato, N., Ashcroft, F. M. & Puljung, M. C. The Nucleotide-Binding Sites of SUR1: A Mechanistic Model. Biophys. J. 109, 2452-2460, doi:10.1016/j.bpj.2015.10.026 (2015).
4. Nichols, C. G. Adenosine Triphosphate-Sensitive Potassium Currents in Heart Disease and Cardioprotection. Card Electrophysiol Clin 8, 323-335, doi:10.1016/j.ccep.2016.01.005 (2016).
5. Bienengraeber, M. et al. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat Genet 36, 382-387, doi:10.1038/ng1329 (2004).
6. Olson, T. M. et al. KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nat Clin Pract Cardiovasc Med 4, 110-116, doi:10.1038/ncpcardio0792 (2007).
7. Harakalova, M. et al. Dominant missense mutations in ABCC9 cause Cantu syndrome. Nat Genet 44, 793-796, doi:10.1038/ng.2324 (2012).
8. Thomas, C. & Tampe, R. Structural and Mechanistic Principles of ABC Transporters. Annu. Rev. Biochem. 89, 605-636, doi:10.1146/annurev-biochem-011520-105201 (2020).
9. Shindo, T., Yamada, M., Isomoto, S., Horio, Y. & Kurachi, Y. SUR2 subtype (A and B)-dependent differential activation of the cloned ATP-sensitive K+ channels by pinacidil and nicorandil. Br J Pharmacol 124, 985-991, doi:10.1038/sj.bjp.0701927 (1998).
10. Matsuoka, T. et al. C-terminal tails of sulfonylurea receptors control ADP-induced activation and diazoxide modulation of ATP-sensitive K(+) channels. Circ. Res. 87, 873-880, doi:10.1161/01.res.87.10.873 (2000).
11. Hambrock, A. et al. ATP-Sensitive K+ channel modulator binding to sulfonylurea receptors SUR2A and SUR2B: opposite effects of MgADP. Mol Pharmacol 55, 832-840 (1999).
12. Schwanstecher, M. et al. Potassium channel openers require ATP to bind to and act through sulfonylurea receptors. EMBO J. 17, 5529-5535, doi:10.1093/emboj/17.19.5529 (1998).
13. Gribble, F. M., Tucker, S. J., Seino, S. & Ashcroft, F. M. Tissue specificity of sulfonylureas - Studies on cloned cardiac and beta-cell K-ATP channels. Diabetes 47, 1412-1418, doi:DOI 10.2337/diabetes.47.9.1412 (1998).
14. Shindo, T., Yamada, M., Isomoto, S., Horio, Y. & Kurachi, Y. SUR2 subtype (A and B)-dependent differential activation of the cloned ATP-sensitive K+ channels by pinacidil and nicorandil. Br. J. Pharmacol. 124, 985-991, doi:10.1038/sj.bjp.0701927 (1998).
15. Matsuoka, T. et al. C-terminal tails of sulfonylurea receptors control ADP-induced activation and diazoxide modulation of ATP-sensitive K(+) channels. Circ. Res. 87, 873-880, doi:10.1161/01.res.87.10.873 (2000).
16. Hambrock, A. et al. ATP-Sensitive K+ channel modulator binding to sulfonylurea receptors SUR2A and SUR2B: opposite effects of MgADP. Mol. Pharmacol. 55, 832-840 (1999).
17. Gribble, F. M., Tucker, S. J., Seino, S. & Ashcroft, F. M. Tissue specificity of sulfonylureas: studies on cloned cardiac and beta-cell K(ATP) channels. Diabetes 47, 1412-1418 (1998).
18. Ding, D. et al. Structural identification of vasodilator binding sites on the SUR2 subunit. Nat Commun 13, 2675, doi:10.1038/s41467-022-30428-y (2022).




