Development of a New Probe for STED Imaging of Mitochondria in Fixed Cells by Zhixing Chen's Research Team in Collaboration with German Scientists
Source: Professor Zhixing Chen's Research Team
On April 30, 2024, the research group led by Professor Zhixing Chen from the College of Future Technology at Peking University, the National Biomedical Imaging Center (NBIC), and the Center for Life Sciences (CLS), in collaboration with the University of Cologne in Germany, published a research paper titled “An Aldehyde-crosslinking Mitochondrial Probe for STED Imaging in Fixed Cells” in Proceedings of the National Academy of Sciences, U.S.A. (PNAS). This study introduced a novel mitochondrial cristae dye, PK Mito Orange Fix (PKMO FX), designed for STED microscopy. PKMO FX enables STED imaging of mitochondria in live cells and, uniquely, retains strong mitochondrial fluorescence after fixation with aldehyde-based fixatives like formaldehyde or glutaraldehyde. This innovation facilitates high-resolution STED imaging (Figure 1) and correlative light and electron microscopy (STED-CLEM) in fixed cells.
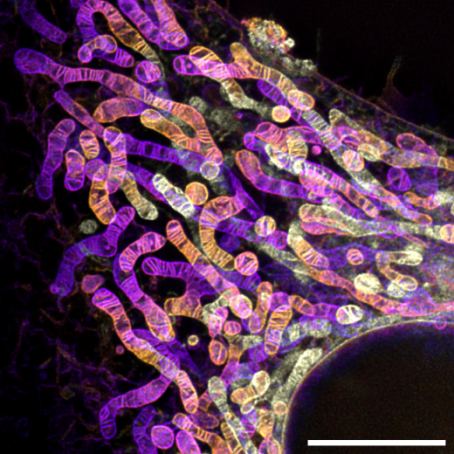
Figure 1. Multilayer STED imaging of mitochondria in glutaraldehyde-fixed HeLa cells labeled with PKMO FX. Scale bar: 5 μm.
Over the past few years, Professor Chen's team has developed the PK Mito series of mitochondrial cristae super-resolution probes (PNAS 2022 cover story; Chem. Sci. 2020), which have been widely adopted worldwide. Since 2022, Dr. Christian Jüngst from the CECAD Imaging Facility at the University of Cologne has utilized PK Mito Orange probes to support cutting-edge research on topics like mitochondria and aging (Nat. Commun. 2022, 6704).
Discussions between Dr. Jüngst and Professor Chen’s team highlighted a unique challenge: live-cell mitochondrial dyes typically lose mitochondrial localization post-fixation, limiting their use in STED imaging of fixed cells. A probe addressing this limitation would enhance compatibility with other fluorescent labeling techniques and promote broader adoption of super-resolution microscopy in cell biology.
Most mitochondrial probes rely on mitochondrial membrane potential (MMP) to drive hydrophobic cations specifically into the mitochondria. Although well implemented in live cells, noncovalent mitochondrial probes will quickly diffuse and lose their localization when fixation causes the rapid dissipation of the MMP. As a result, they diffuse to nearby lipid structures, such as the endoplasmic reticulum, causing low brightness and poor signal-to-noise ratios in fixed-cell imaging. Conventional approaches, like attaching reactive groups (e.g., benzyl chloride in Mito Tracker dyes), allow covalent binding to protein nucleophilic residues but suffer from low cross-linking efficiency and poor imaging performance. Moreover, the outdated optical properties of the MitoTracker series fail to meet the requirements for STED imaging, particularly for fixed cells.
Building on their previous work with PK Mito Orange, the researchers utilized the Cy3.5 dye, optimized for mitochondrial STED imaging, to develop a fixable mitochondrial probe. By attaching a primary amine to Cy3.5, the team leveraged aldehyde-mediated condensation during fixation to achieve efficient cross-linking with proteins and fixatives. This new probe, named PKMO FX, employs a "fixation-driven chemical cross-linking" strategy (Figure 2).
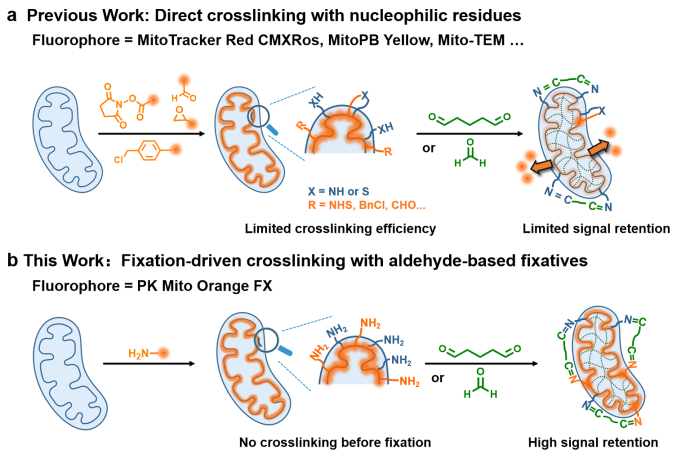
Figure 2. Comparison of cross-linking strategies between PKMO FX and traditional fixable mitochondrial probes.
PKMO FX demonstrated superior fluorescence retention and signal-to-noise ratios post-fixation compared to existing probes. STED imaging of fixed cells labeled with PKMO FX revealed clear mitochondrial cristae at a resolution of approximately 70 nm. Z-axis multi-layer STED imaging highlighted the diverse morphologies of mitochondria. PKMO FX proved effective across various cell types, including cancer cells, cardiomyocytes, neurons, and protein-deficient cells.
Additionally, PKMO FX was compatible with other fixation-compatible labeling methods, such as fluorescent protein expression, self-labeling protein tags, co-staining with other fixable dyes, metabolic labeling, and immunofluorescence. This enabled multi-color imaging of cellular structures in fixed samples (Figure 3).
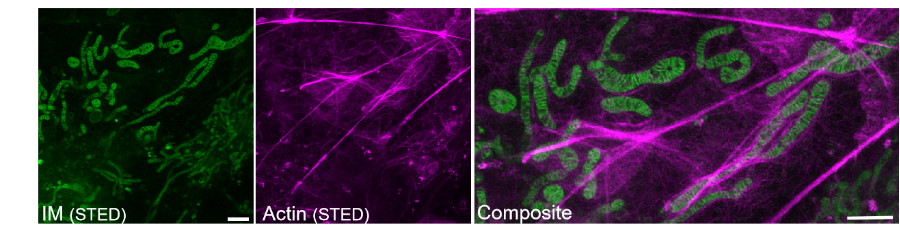
Figure 3. PKMO FX-labeled mitochondrial cristae and immunolabeled actin. Scale bar: 1 μm.
PKMO FX enabled visualization of mitochondrial ultrastructure in electron microscopy (EM) images. Combining SYBR Gold-stained mtDNA with PKMO FX-labeled mitochondrial cristae, the team achieved dual-color CLEM, precisely localizing mtDNA within mitochondrial cristae (Figure 4). The study revealed that mtDNA predominantly resides in large cavities of cristae, a feature challenging to observe with EM alone. This innovation bridges fluorescence and EM, providing a powerful tool for CLEM studies.

Figure 4. Dual-color CLEM imaging of mitochondrial cristae and mtDNA using PKMO FX. Scale bar: 3 μm.
In summary, the authors employed an emerging bioconjugation strategy to develop an aldehyde-fixable mitochondrial probe. PKMO FX combines excellent optical properties with robust fluorescence retention post-fixation, enabling visualization of mitochondrial cristae ultrastructure in fixed cells for the first time using small-molecule dyes.
Jingting Chen, a Ph.D. candidate at the College of Future Technology, Peking University, is the first author of this paper. Zhixing Chen, a researcher at the College of Future Technology, Peking University, the NBIC, and the CLS, and Christian Jüngst from the University of Cologne are the co-corresponding authors. This work also benefited from collaboration with Professor Stefan Jakobs’ group at the University of Göttingen/Max Planck Institute. Since its establishment in 2018, Professor Chen Zhixing's team has focused on developing cutting-edge fluorescent probes to integrate cell biology with next-generation imaging technologies.




