Novel Dual-Objective Two-Photon Microscope Configuration Developed by the Team of Heping Cheng and Aimin Wang Extends the Limits of Imaging Depth
Source: Heping Cheng and Aimin Wang's Research Team
Long-term, high-resolution optical 3D imaging of living biological samples is crucial for exploring physiological functions and systemic behaviors at mesoscopic and microscopic levels. However, due to the high scattering properties of biological tissues, high-resolution optical imaging is often confined to superficial layers of samples. Multiphoton microscopy (MPM), leveraging longer excitation wavelengths and nonlinear excitation properties, offers greater imaging penetration depth, making it widely applicable in deep imaging of highly scattering biological samples. Nevertheless, deep imaging with multiphoton microscopy typically requires higher excitation power or prolonged exposure, which can induce photodamage and limit its use in long-term imaging.
To enable long-term, large-volume, high-resolution 3D imaging of thick, highly scattering biological samples, the research team led by Professors Heping Cheng and Aimin Wang at Peking University developed the Dual-Objective Two-photon Microscope (Duo-2P). Their work, titled "Dual-objective two-photon microscope for volumetric imaging of dense scattering biological samples by bidirectional excitation and collection," was published in Photonics Research.
To accommodate the properties of small, dense, and highly scattering samples such as tissue slices, organoids, developing embryos, and small organisms, the research team developed a dual-objective, dual-sided two-photon microscope configuration (Figures 1 and 2). In this design, upright and inverted resonant-scanning two-photon microscopes are symmetrically positioned on opposite sides of the sample. Each microscope is responsible for imaging half of the sample volume. Layer-by-layer imaging is achieved through laser path switching and axial stepping of the objectives, and the complete 3D image is synthesized from the volumetric data.
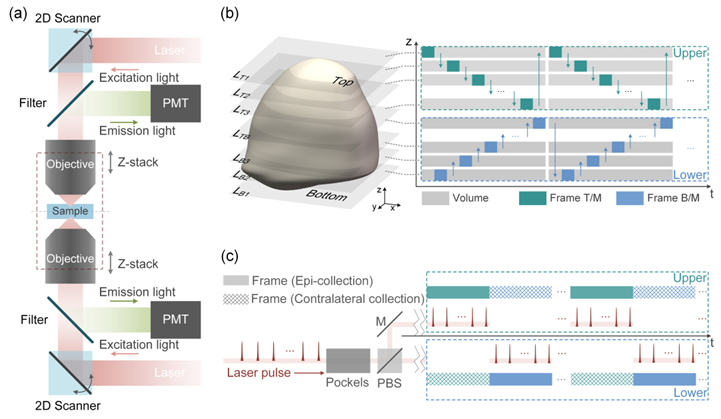
Figure 1. (a) Configuration of Duo-2P. (b) Alternate volumetric imaging by Duo-2P, one frame at a time. Z-stack images from the two microscopes are combined to synthesize a full 3D image of the sample. (c) The excitation and fluorescence collection timing of Duo-2P. Epi- and contralateral fluorescent signals are collected simultaneously by the two microscopes, detected by respective PMT, and digitally combined to form a frame image.

Figure 2. Duo-2P Imaging System
By innovatively employing a compact dual-objective two-photon microscope configuration, Duo-2P extends the maximum imaging depth (limited by the signal-to-background ratio) to more than twice that of conventional systems. For samples with a thickness five times the scattering length, Duo-2P reduces total excitation energy by an order of magnitude compared to traditional epi-2P microscopes. Furthermore, fluorescence collection from the opposite side enhances image quality, particularly in deeper sample regions, where the signal-to-noise ratio (SNR) improves by up to 1.4-fold. The dual-objective configuration also increases the imaging volume, overcoming the working distance limitations of single-objective systems. In low-scattering thick samples, the Duo-2P achieves complete volumetric imaging of biological specimens with thickness twice the working distance of the objectives. The team validated the Duo-2P's imaging performance through simulations and experimental analyses (Figure 3). Combined with a dual-sided imaging chamber compatible with Duo-2P, the system successfully recorded calcium signals from thousands of neurons in the highly scattering, densely labeled suprachiasmatic nucleus (SCN) of mice for four continuous hours.

Figure 3. Simulations of bidirectional excitation and fluorescence collection in Duo-2P. (a) Enlarged schematic of the imaging chamber outlined by the dashed box in Fig. 1(a). (b) Comparison of the excitation laser power for Duo-2P and epi-2P imaging with the same SNR at a sample thickness four times the scattering length. (c) Ratios of epi-2P’s excitation energy input to that of Duo-2P imaging across different sample thicknesses. (d) Effects of imaging depth (D) and defocus distance (Δd) on fluorescence collection efficiency (η). (e) Simulated SNR in relation with the contralateral collection and fluorescence intensity.
In research on mammalian biological clock time coding, Duo-2P enabled circadian-scale calcium imaging of nearly 10,000 neurons, providing comprehensive data to elucidate the SCN's time-coding mechanisms. In the future, this achievement is expected to offer significant technical support for studies in neuroscience, developmental biology, and organoid research.
Muyue Zhai, a Ph.D. candidate at the College of Future Technology, Peking University, is the first author of the paper. Professor Heping Cheng from the College of Future Technology and the National Biomedical Imaging Center (NBIC), Peking University, and Professor Aimin Wang from the School of Electronics and NBIC, Peking University, are the corresponding authors. This work was supported by grants from the National Natural Science Foundation of China and the CAMS Innovation Fund for Medical Sciences.




