Chen Lei's team reported the mechanism of recognition of small molecule inhibitors and activators by human UCP1
Information source: Chen Lei researcher's team
On June 19, 2023, Chen Lei research group from the Molecular Medicine Research Institute of Peking University's Future Technology School, the Peking University-Tsinghua University Joint Center of Life Science, and the National Biomedical Imaging Center, published an article in the journal Nature titled "Structural basis for the binding of DNP and purine nucleotides onto UCP1." The article reports high-resolution cryo-electron microscopy structures of human UCP1 in three states: nucleotide-free binding, DNP binding, and ATP binding. You can find the article at https://www.nature.com/articles/s41586-023-06332-w.
Mitochondria are the primary sites for aerobic respiration within cells and serve as the "powerhouses" of cellular metabolism. The electron transport chain on the inner mitochondrial membrane simultaneously pumps protons while transferring electrons from reducing coenzymes to oxygen. This creates a proton gradient across the inner mitochondrial membrane, driving the rotation of ATP synthase to generate ATP1. During this process, the low permeability of the mitochondrial inner membrane to protons ensures a tight coupling between electron transport and ATP production. Brown adipose tissue is an important thermogenic organ in mammals, with high expression of Uncoupling Protein 1 (UCP1) located on the inner mitochondrial membrane. When exposed to cold stimuli, UCP1 is activated and transports protons, uncoupling electron transport from ATP production, thereby converting the proton gradient into heat release2,3. Increasing evidence suggests that activating brown adipose tissue thermogenesis can effectively combat obesity and related metabolic disorders4,5 and holds clinical promise in cancer therapy6.
UCP1 serves as the ultimate effector molecule in the thermogenesis process, and its activity is tightly regulated. Under normal conditions, UCP1 activity is inhibited by high concentrations of purine nucleotides in the cytoplasm (primarily ATP under physiological conditions). When exposed to cold stimuli, the sympathetic nervous system releases norepinephrine, which acts on brown adipocytes, promoting lipolysis. The resulting fatty acids can overcome the inhibitory effect of ATP and activate UCP1 to generate heat2,3. Furthermore, some synthetically engineered chemical compounds, including 2,4-dinitrophenol (DNP), can effectively activate UCP17. However, it remains unclear how DNP and ATP bind to UCP1 and regulate its activity.
UCP1 is a membrane protein with a molecular weight of only ~32 kDa and no apparent soluble structural domains. Directly resolving its structure using cryo-electron microscopy presents significant challenges. Therefore, the authors screened a library of artificially synthesized nanobodies (sybodies)8,9 to obtain one that recognizes UCP1. They further increased the molecular weight using the legobody10 strategy. After overcoming a series of difficulties, including protein purification, nanodisc reconstitution, cryo-sample preparation, and data processing, the authors ultimately achieved electron density maps at resolutions of 2.51 Å to 2.57 Å for UCP1 in three states: nucleotide-free binding, DNP binding, and ATP binding. They also constructed atomic models.

Figure 1: Structure and topology diagram of UCP1
UCP1 belongs to the mitochondrial carrier family, a six-transmembrane transport protein composed of three structurally similar repeats. Each repeat consists of two transmembrane helices and a short helix on the matrix side. Notably, the odd-numbered transmembrane helices contain conserved prolines and exhibit significant kinks (Figure 1). Additionally, the authors observed a close association between cardiolipin (CDL) and UCP1 (Figure 1). The authors found that UCP1 in the nucleotide-free binding state adopts an open conformation on the cytoplasmic side (c-state), with a central cavity containing a higher positive charge density (Figure 2). This feature facilitates its binding with negatively charged purine nucleotides on the cytoplasmic side.
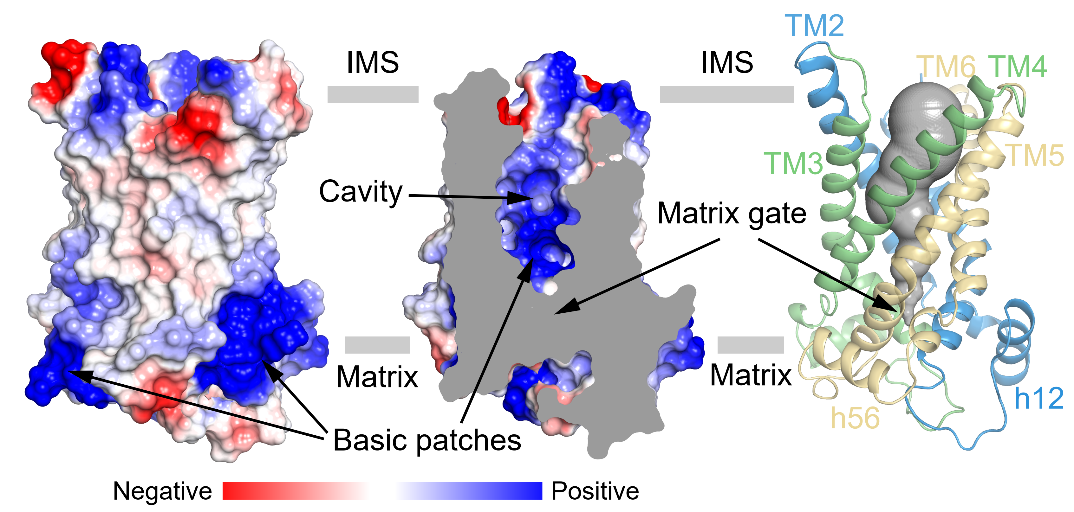
Figure 2: UCP1 is in an open conformation on the cytoplasmic side
UCP1 in the DNP-bound state also adopts the c-state conformation, with an overall structure similar to that of UCP1 in the nucleotide-free binding state. DNP binds within the central cavity of UCP1, between TM2 and TM6, through π-π interactions as well as hydrophobic and electrostatic interactions. This binding induces conformational changes in R91 and R276 (Figure 3).

Figure 3: Binding sites of DNP
The binding site of ATP to UCP1 is similar to that of DNP, located within the central cavity of UCP1 and involving close interactions with multiple transmembrane segments. ATP's adenine moiety engages in a cation-π interaction with R91 of TM2, causing TM2 to bend inward and leading to conformational changes in TM1, TM4, TM5, and TM6. This transition results in UCP1 adopting a cytoplasmic-side-closed, more compact conformation (Figure 4). In addition to R91, ATP binding also induces conformational changes in R83 and R276 (Figure 4). Furthermore, the binding sites of ATP and DNP directly overlap in space, explaining why ATP can inhibit DNP-mediated activation of UCP1.
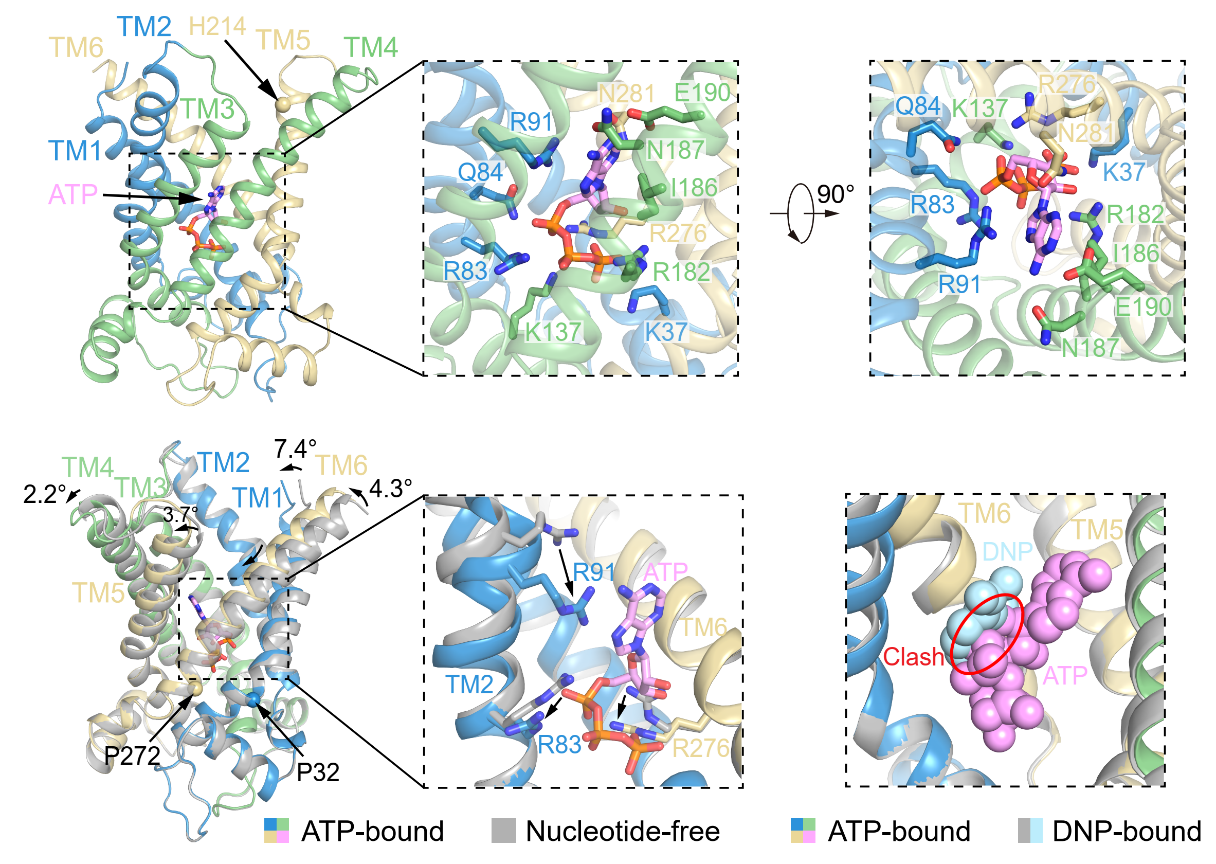
Figure 4: ATP binding sites
In summary, the authors elucidated the structures of UCP1 in various states using cryo-electron microscopy. They observed at the atomic level how DNP and ATP bind to UCP1 and induce conformational changes. This work establishes a structural foundation for a deeper understanding of the functioning mechanism of UCP1.
The first author of this study is Dr. Yunlu Kang from the research group of Dr. Lei Chen, who is also the second author and corresponding author. Valuable input for the execution of this research was provided by Dr. Yifu Qiu and Dr. Kaili Xue from the Molecular Medicine Institute of the Future Technology Institute at Peking University. Professor Markus Seeger from the University of Zurich supplied the mRNA library for sybodies. Professor Dianfan Li and Dr. Tingting Li from the Institute of Biochemistry and Cell Biology, Shanghai, offered advice on sybody screening. This work was supported by funding from the Key Research and Development Program of the Ministry of Science and Technology, the National Natural Science Foundation of China, and the Joint Center for Life Sciences. Dr. Yunlu Kang received support from Peking University's Boya Postdoctoral Fellowship. The preparation, screening, and data collection for cryo-electron microscopy in this project were carried out at Peking University's Cryo-EM Platform and Electron Microscopy Facility, with assistance from Xue Mei Li, Zhenxi Guo, Xia Pei, Changdong Qin, Xiaojuan Hui, Guopeng Wang, and others. Data processing for this project received hardware and technical support from Peking University's CLS Computing Platform and WEMIND Supercomputing Platform.
References:
1. Schultz, B. E. & Chan, S. I. Structures and proton-pumping strategies of mitochondrial respiratory enzymes. Annu Rev Biophys Biomol Struct30, 23-65, doi:10.1146/annurev.biophys.30.1.23 (2001).
2. ivakaruni, A. S. & Brand, M. D. The regulation and physiology of mitochondrial proton leak. Physiology (Bethesda)26, 192-205, doi:10.1152/physiol.00046.2010 (2011).
3. houchani, E. T., Kazak, L. & Spiegelman, B. M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab29, 27-37, doi:10.1016/j.cmet.2018.11.002 (2019).
4. Becher, T. et al. Brown adipose tissue is associated with cardiometabolic health. Nature medicine27, 58-65, doi:10.1038/s41591-020-1126-7 (2021).
5. Cypess, A. M. Reassessing Human Adipose Tissue. The New England journal of medicine386, 768-779, doi:10.1056/NEJMra2032804 (2022).
6. Seki, T. et al. Brown-fat-mediated tumour suppression by cold-altered global metabolism. Nature608, 421-428, doi:10.1038/s41586-022-05030-3 (2022).
7. Bertholet, A. M. et al. Mitochondrial uncouplers induce proton leak by activating AAC and UCP1. Nature606, 180-187, doi:10.1038/s41586-022-04747-5 (2022).
8. Zimmermann, I. et al. Synthetic single domain antibodies for the conformational trapping of membrane proteins. Elife7, doi:10.7554/eLife.34317 (2018).
9. Zimmermann, I. et al. Generation of synthetic nanobodies against delicate proteins. Nat Protoc15, 1707-1741, doi:10.1038/s41596-020-0304-x (2020).
10. Wu, X. & Rapoport, T. A. Cryo-EM structure determination of small proteins by nanobody-binding scaffolds (Legobodies). Proceedings of the National Academy of Sciences of the United States of America118, doi:10.1073/pnas.2115001118 (2021).




