A powerful tool for deep brain imaging: the miniaturized three-photon microscope of Peking University is launched
Source of Information: Prof. Cheng Heping - Prof. Wang Aimin's Team
On February 23, 2023, the team of Heping Cheng and Aimin Wang from Peking University published an article titled "Miniature three-photon microscopy maximized for scattered fluorescence collection" in Nature Methods online (link: https://doi.org/10.1038/s41592-023-01777-3). The article reports on a miniature three-photon microscope weighing only 2.17 grams (Figure 1), which enables functional imaging of the entire cortex and hippocampal neurons in freely behaving mice for the first time. This opens up a new research paradigm for uncovering the neural mechanisms in deep brain structures.
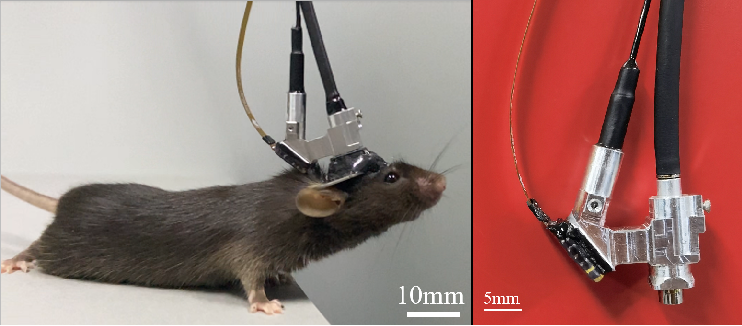
Figure 1 Picture of mice wearing a miniaturized three-photon microscope
Mapping the connectome and functional dynamics of the brain is a key research focus of China and several other countries' brain initiatives, which requires the development of wearable microscopic imaging tools for freely moving animals. In 2017, the team of Academician Cheng Heping from Peking University successfully developed the first-generation 2.2-gram miniature two-photon microscope, which obtained dynamic images of cortical neurons and synaptic activity in the brains of freely behaving mice. In 2021, the team's second-generation miniature two-photon microscope expanded the imaging field of view by 7.8 times and enabled three-dimensional imaging of thousands of neuronal functional signals in the cortex.
The latest miniature three-photon microscope from Peking University has overcome the imaging depth limitations of previous miniature multi-photon microscopes. The excitation light path of the microscope can penetrate the entire mouse cortex and corpus callosum, enabling direct observation and recording of the hippocampal CA1 subregion in mice (Figure 2, Video 1-2). The maximum imaging depth for neuronal calcium signals is 1.2 mm, and for vascular imaging, it is 1.4 mm. Furthermore, in terms of phototoxicity, cortical calcium signal imaging only requires a few milliwatts, and hippocampal calcium signal imaging only requires 20 to 50 milliwatts, which is significantly lower than the safe threshold for tissue damage. Therefore, this miniature three-photon microscope can continuously observe neuronal functional activity for long periods without causing significant photobleaching or photodamage.
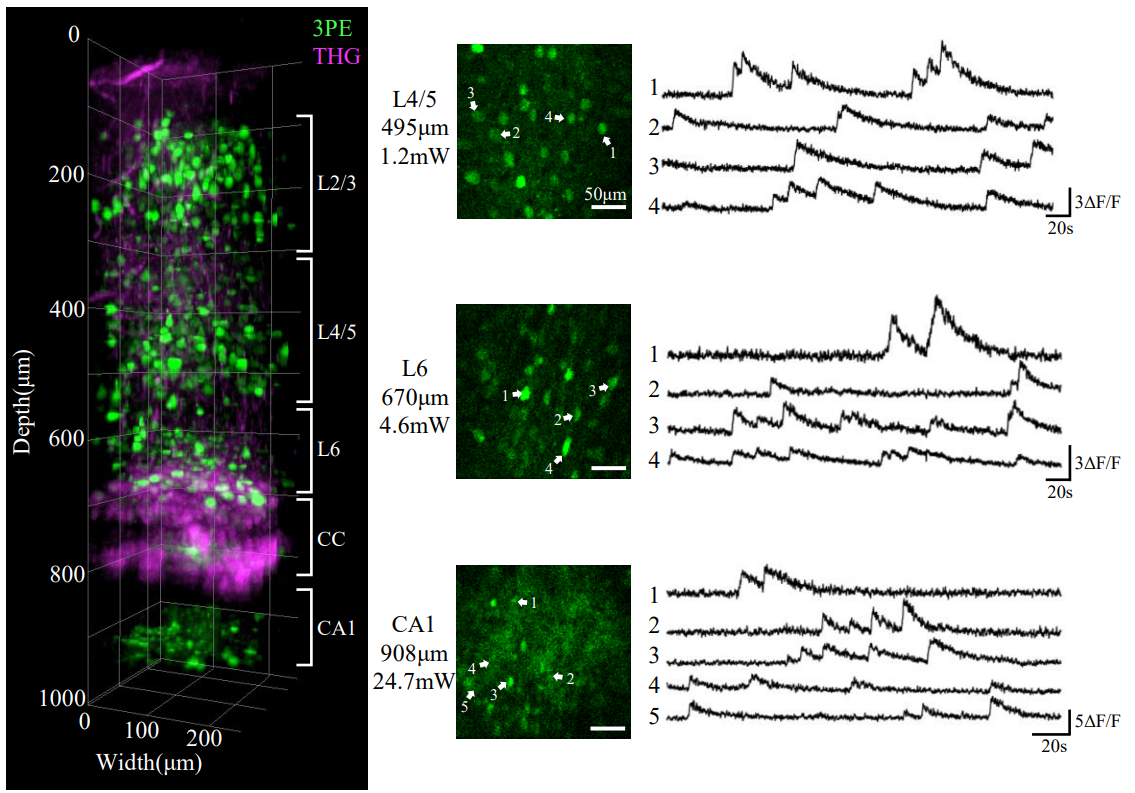
Figure 2 shows the structural and functional dynamics of the mouse cortex (L1-L6) and hippocampal CA1 recorded using the miniature three-photon microscopy. CC: Corpus callosum. The green color represents the fluorescence calcium signals of neurons labeled with GCaMP6s, and the magenta color represents the third harmonic signals from the dura mater, microvessels, and brain-white matter interface.
Video 1 shows a three-dimensional reconstruction of the mouse brain from the cortex to the corpus callosum and then to the hippocampal CA1 subregion, captured using the miniature three-photon microscope from Peking University. The green color represents the fluorescence signals of neurons labeled with GCaMP6s, and the magenta color represents the third harmonic signals from the dura mater, microvessels, and brain-white matter interface. The top left corner displays the imaging depth, as the laser enters the brain, starting from the dura mater as the reference point, moving downward along the z-axis stage, revealing the layered neuronal cell bodies and microvessels in the cortex L1 to L6, followed by the dense fiber structure of the corpus callosum. After passing through the corpus callosum, further downward movement reveals the neuronal cell bodies in the hippocampal CA1 subregion.
Video 2 The bottom left image shows a mouse wearing a miniaturized three-photon probe, freely exploring in a cage (29 cm long × 17.5 cm wide × 15 cm high). The top left image shows the miniaturized three-photon probe worn by the mouse imaging the fluorescence calcium signals of neurons in the hippocampal CA1 subregion at a depth of 978 μm (frame rate 8.35 Hz, optical power behind the objective lens is 35.9 mW). The right image shows the calcium activity traces of 10 neurons in the top left image, with peaks representing calcium signal firing. The blue line moving along the calcium activity traces is synchronized with the mouse's free behavior video.
The hippocampus is located beneath the cortex and the corpus callosum, and plays an important role in the consolidation of short-term to long-term memory, spatial memory, and emotional encoding. In rodent research models, the hippocampus is more than one millimeter deep from the brain surface. Due to the high scattering optical properties of brain tissue, especially the corpus callosum, breaking through the imaging depth limit has been a major challenge for neuroscientists for a long time. Previous miniaturized single-photon and multi-photon microscopes were unable to achieve non-destructive imaging of the hippocampal region by directly penetrating the entire cortex.
The breakthrough in the imaging depth of the three-photon microscope at Peking University is attributed to a novel optical design (Figure 3). By simulating a layered scattering model of the cortex, white matter, and hippocampus, the authors found that the fluorescence signal becomes randomly scattered when it reaches the brain surface from deep tissues, leading to a decrease in the collection efficiency of the microscope objective and greatly limiting the imaging depth. To address this issue, the classical Abbe condenser structure is introduced into the design: a miniaturized Abbe condenser combined with a simplified infinity-corrected objective improves the transmission efficiency of scattered light; the Abbe condenser is also reused with the microprism in the excitation light path, further simplifying the structure and reducing losses. Overall, the scattering fluorescence collection efficiency of the new miniaturized microscope has achieved a multiple-fold improvement.
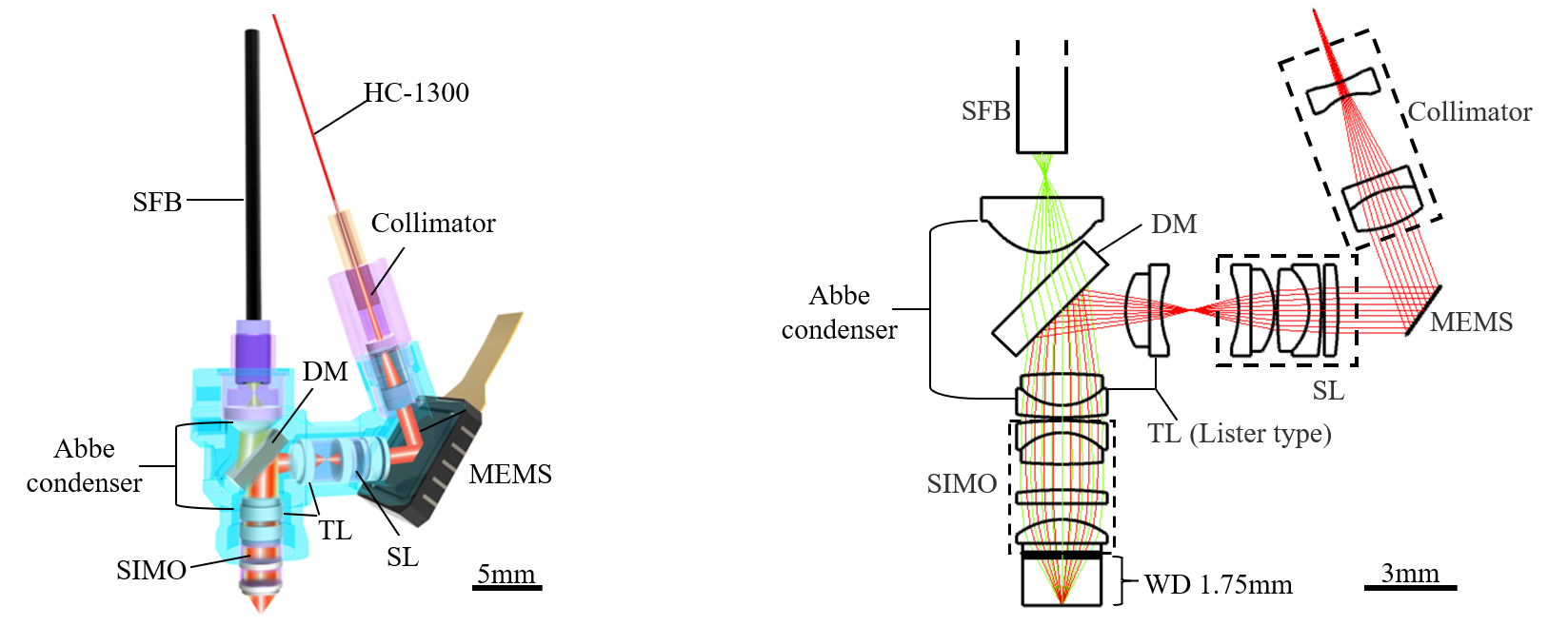
Figure 3: Optical configuration of miniaturized three-photon microscope
Meanwhile, using the miniaturized three-photon microscope, the authors investigated the encoding mechanism of layer 6 neurons in the mouse somatosensory cortex during the process of grasping a sugar bean. They found that approximately 37% of neurons were active before the grasping action and most active during grasping, while approximately 5.6% of neurons became active after the grasping action, indicating the involvement of different neurons in different stages of encoding (Figure 4, Video 3). These results provide a preliminary demonstration of the potential application of the miniaturized three-photon microscope in neuroscience research.
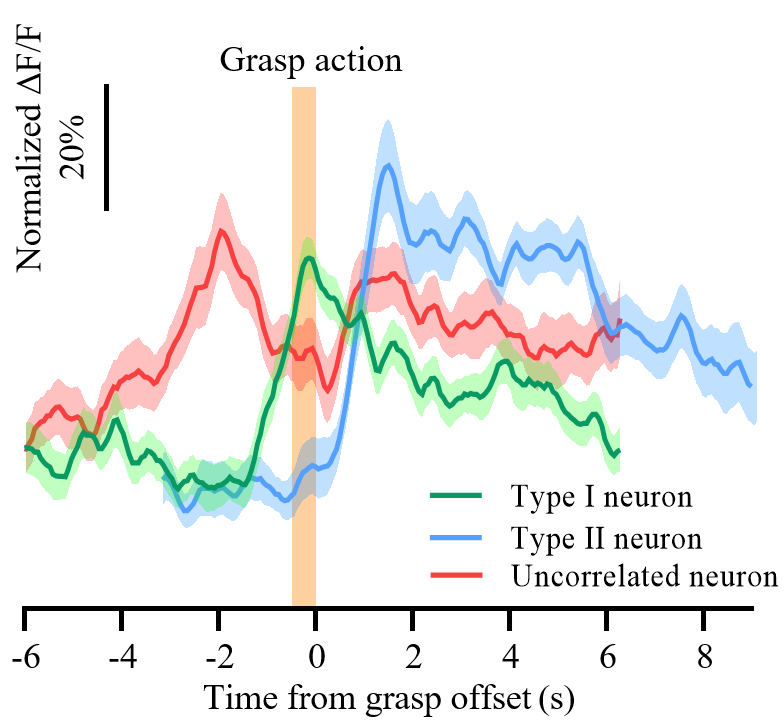
Figure 4 Different response types of layer 6 neurons in the mouse somatosensory cortex during the grasping sugar bean task.
Video 3 The left image shows a mouse wearing a miniaturized three-photon microscope, grabbing and eating candy beans through a 0.5 cm slit. The middle image is a fluorescence calcium signal (neurons labeled with GCaMP6s) captured by the probe of the miniaturized three-photon microscope in the PPC brain region, layer 6 neurons (at a depth of 650 micrometers) with a frame rate of 15.93 Hz. The right image shows the calcium activity traces of five neurons selected from the middle image, with each green line representing a mouse's grabbing action. The moving blue line is synchronized with the mouse's behavior video in the left image and the neuron activity in the middle image. The video is played at normal speed (×1), slow speed (×0.5), and fast speed (×10) for easy viewing of the grabbing behavior.
Dr. Zhao Chunzhu, a postdoctoral researcher at the Future Technology Institute of Peking University, Chen Shiyuan, a doctoral student at the Interdisciplinary Research Institute of Peking University, and Dr. Zhang Lifeng, a researcher at the Nanjing Translational Research Institute of Molecular Medicine, Chinese Academy of Medical Sciences, are the co-first authors of this paper. Prof. Cheng Heping, Prof. Wang Aimin, and Dr. Zhao Chunzhu from Peking University are the corresponding authors of the paper. This project was supported by funding from the 2030 Science and Technology Innovation Project "Brain Science and Brain-like Research," the Innovative Unit of Mitochondrial Mechanisms of Brain Diseases of the Medical and Health Science and Technology Innovation Project of the Chinese Academy of Medical Sciences, the National Natural Science Foundation of China, the National Major Scientific Research Instruments Development Project, and the Key Research and Development Program of the Ministry of Science and Technology.




