A live-cell quantitative high-fidelity super-resolution method has been developed by Chen Lab
Source of Information: Chen Liangyi Lab
On May 30th, 2023, Chen Liangyi's research group at College of Future Technology School of Peking University published a research paper titled "Quantitative structured illumination microscopy via a physical model-based background filtering algorithm reveals actin dynamics" in Nature Communications. The research group carefully studied the imaging process of structured illumination microscopy and proposed an algorithm based on a physical imaging model to distinguish between in-focus signals and out-of-focus background, successfully removing background fluorescence. By further incorporating the previously developed sparse deconvolution algorithm, they achieved high-fidelity super-resolution imaging of live cells with a resolution better than 70nm while preserving the integrity of weak fluorescence signals and linearity of reconstructed signals. The paper can be found at: https://www.nature.com/articles/s41467-023-38808-8.
Maintaining the integrity of imaging structures while improving the spatial and temporal resolution of SIM and eliminating noise and artifacts has always been a core issue in super-resolution imaging. In 2018, the research group found that SIM is susceptible to interference from amplified random noise during Wiener deconvolution. To address this issue, they proposed using the spatiotemporal continuity prior of biological samples to reduce noise artifacts. In 2022, the research group developed a sparse deconvolution algorithm that utilizes the sparsity of fluorescence images as another universal prior knowledge. This algorithm improves the efficiency of photon-number resolution conversion from a mathematical perspective, further enhancing the spatiotemporal resolution capability of super-resolution imaging of live cells, achieving a sampling rate of 564Hz and a resolution better than 70nm.
However, despite the significant improvement in resolution and contrast achieved by sparse deconvolution, the research team found that periodic artifacts appeared in the processed Wiener-SIM super-resolution images due to the prior knowledge of spatiotemporal continuity. This is because periodic artifacts also satisfy the prior knowledge of spatiotemporal continuity and sparsity, so if these artifacts are not completely eliminated by Wiener reconstruction, subsequent sparse deconvolution will amplify these artifacts. These significant periodic artifacts are caused by high-intensity or unevenly distributed background fluorescence. During Wiener-SIM reconstruction, low-frequency background signals are incorrectly shifted to high-frequency components, resulting in periodic artifacts. To solve this problem, there are currently two approaches: one is to directly separate the background fluorescence component from the original image, and the other is to use a notch filter to remove specific frequency components in the spectrum. However, both methods face challenges: separating the background from the original image needs to avoid weak signals being eliminated during super-resolution reconstruction, and using a notch filter method (such as NF-SIM, HiFi-SIM) requires empirical adjustment of parameters for images of different organelles, even if these images are captured using the same microscope system parameters. In addition, both methods may introduce non-linear changes in signal intensity, making the interpretation of fluorescence signals more complex.
To address these issues, they proposed four criteria for quantitatively high-fidelity super-resolution imaging of live cells:
Transparency and reproducibility: Ensuring that different researchers can obtain the same results under the same parameters;
Minimization (or elimination) of reconstruction artifacts and illusions: Ensuring the accuracy and reliability of reconstructed structures;
Balance between spatial resolution and fluorescence structure integrity: Efficient super-resolution methods should provide high resolution while maintaining weak signals;
Linearity of fluorescence signal after reconstruction: Emphasizing the importance of accurate quantification of fluorescence signals.
To address these issues, researchers have proposed methods based on physical imaging models to remove the background without affecting the true signal. Since the images captured by the camera are obtained by convolving the fluorescence excited at different sample depths with the corresponding point spread function (PSF), it is possible to consider separating the convolved in-focus signal from the out-of-focus background (see Figure 1). By approximating the out-of-focus sample distribution using the in-focus sample distribution, researchers have obtained a new SIM imaging model.
![]()
Among them, ![]() is the sine illumination mode,
is the sine illumination mode, ![]() and
and ![]() is PSF in the intra-focal range and extra-focal range, respectively.
is PSF in the intra-focal range and extra-focal range, respectively.
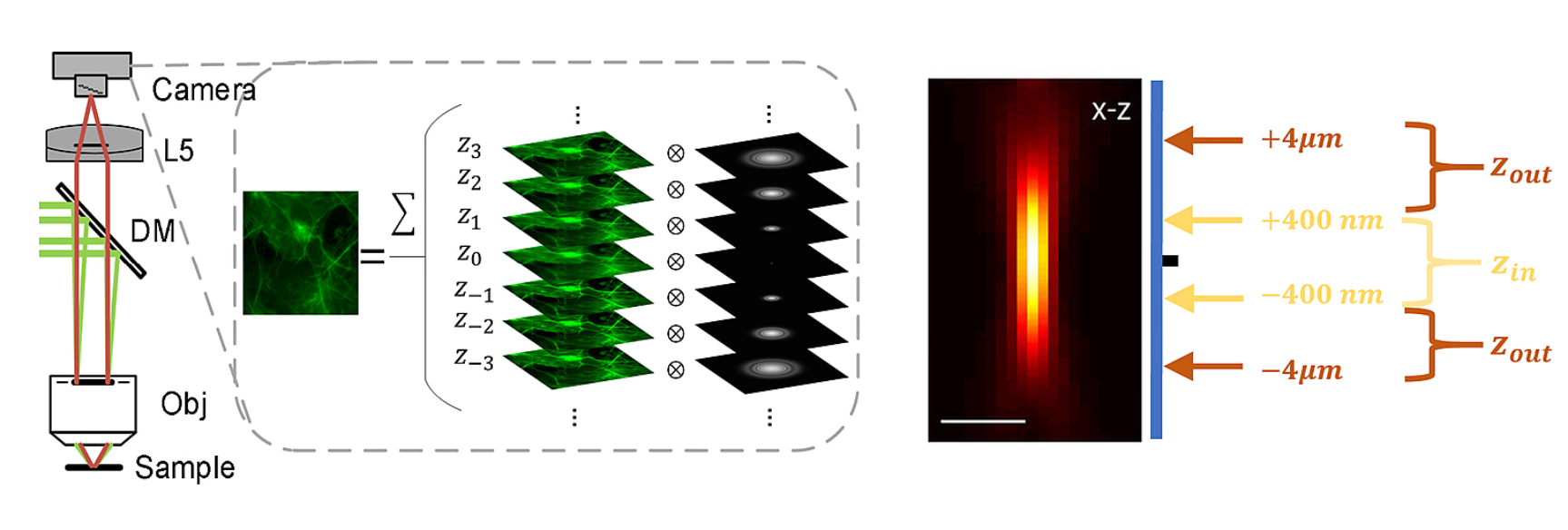
Figure 1. The SIM original image is obtained by overlaying fluorescence at different depths and illustrating the differentiation between in-focus and out-of-focus regions along the axial direction through the profile of the point spread function (PSF).
By introducing this new background filtering (BF) method, BF-SIM successfully eliminated erroneous feature components in the actin spectrum and reduced periodic artifacts in Wiener-SIM and Sparse-SIM images. In addition, compared to Wiener-SIM and HiFi-SIM, BF-SIM performs better, achieving higher contrast super-resolution images of actin fibers, while also providing more reliable detection of weak signals from actin filaments (see Figure 2). On the other hand, the linearity of the lipid droplet SR images reconstructed by BF-SIM is comparable to that of Wiener-SIM, and better than HiFi-SIM (Figure 2).
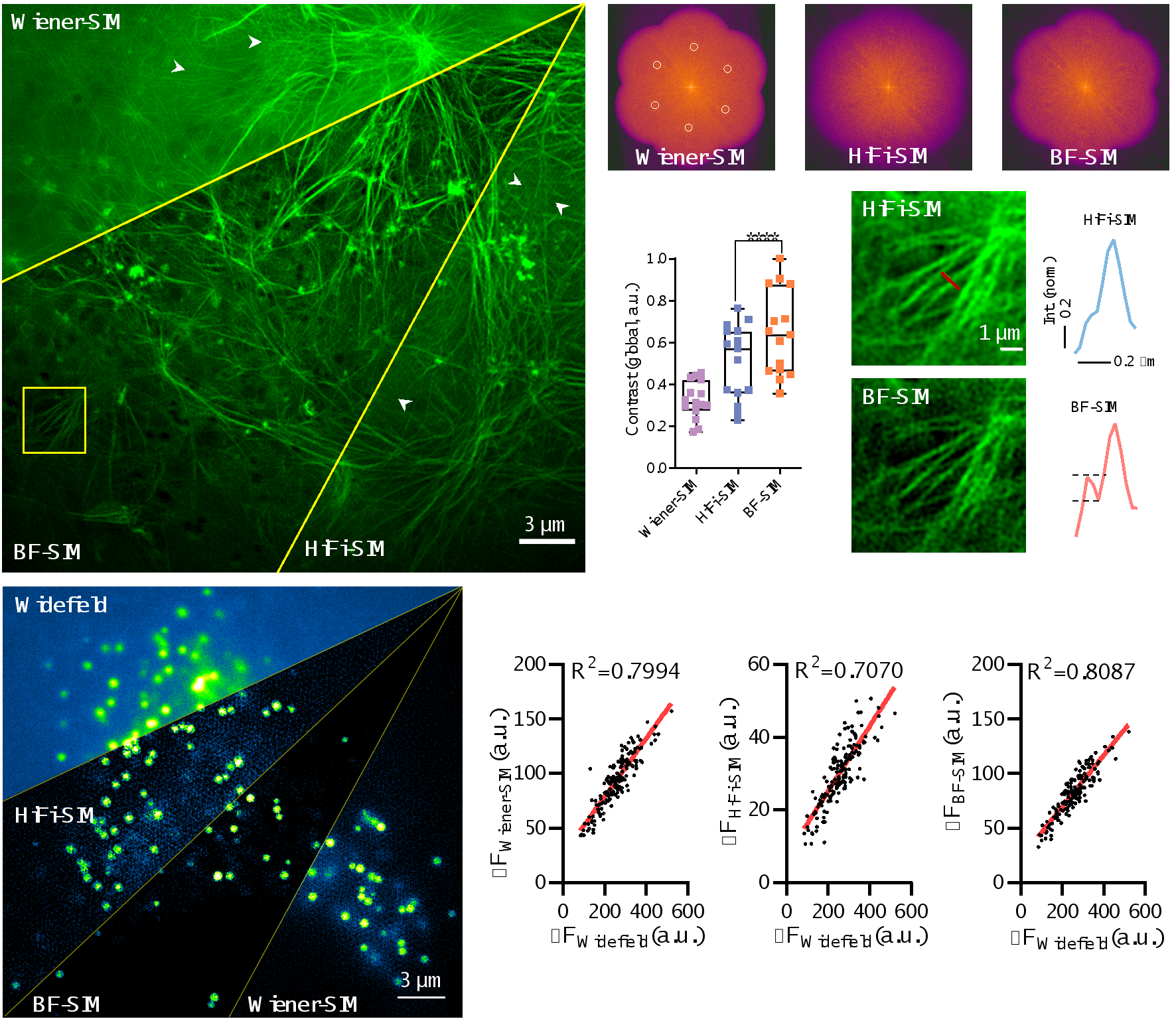
Figure 2. BF preprocessing removes erroneous feature points in the spectrum, suppresses artifacts from fixed patterns, and maintains the integrity of weak signals. Additionally, the linearity of signal reconstruction in BF-SIM is superior to HiFi-SIM.
Finally, the paper used densely labeled actin mesh structures to compare the performance of BF-Sparse-SIM with Sparse-SIM using wavelets to eliminate the background (Wavelets-Sparse-SIM) (see Figure 3, video 1). Although both methods achieve resolutions better than 70 nm, the fluctuation variance of the fluorescence intensity of actin fibers in BF-Sparse-SIM is only half to a quarter of that in Wavelets-Sparse-SIM, indicating that BF-Sparse-SIM has higher continuity in processing weak signals. Furthermore, after skeletonizing the super-resolution images reconstructed by BF-Sparse-SIM, the measured actin fiber density and average length are 1.5 times and 2.5 times that of Wavelets-Sparse-SIM, respectively.
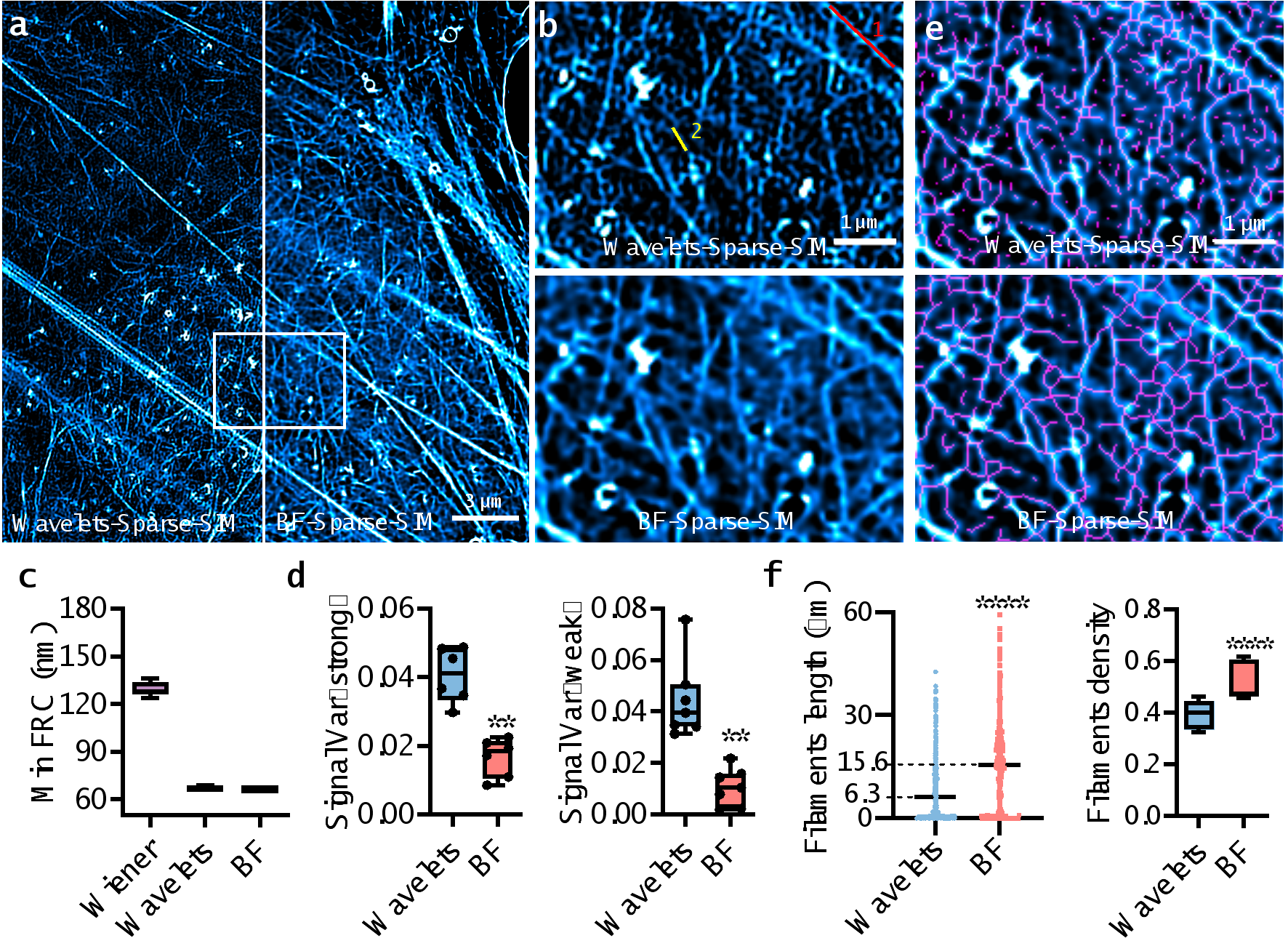
Figure 3. BF-Sparse-SIM exhibits higher fidelity and a more complete structure of the actin network compared to Wavelets-Sparse-SIM.
Video 1. Comparison of the reconstruction of densely labeled actin using BF-Sparse-SIM and Wavelets-Sparse-SIM.
Thanks to the high-fidelity quantification of BF-Sparse-SIM, this paper discovered three new local dynamic processes of actin filaments on the surface of RAW 264.7 cells (see Figure 4). 1) "Actin blip", which appears at the nodes of the actin network, has a small distribution area, and the fluorescence intensity rapidly rises and then quickly falls. 2) "Actin cloud", which starts at an actin aggregation point, gradually attracts more actin, and then the fluorescence signal slowly spreads along the cell skeleton. The distribution area of Actin cloud is larger, and the process of fluorescence intensity rise and decay is also slower. In some cases, some Actin cloud events have a plateau period when the fluorescence intensity reaches its maximum, and then slowly decay. 3) "Actin vortex", where the outer ring fluorescence first brightens, and then gradually brightens in a spiral manner from the outside to the inside. After the fluorescence intensity reaches its maximum, the fluorescence gradually decays. Interestingly, half of the Actin vortex events have two fluorescence enhancement rates: first a slow rise phase, and then a rapid rise phase.
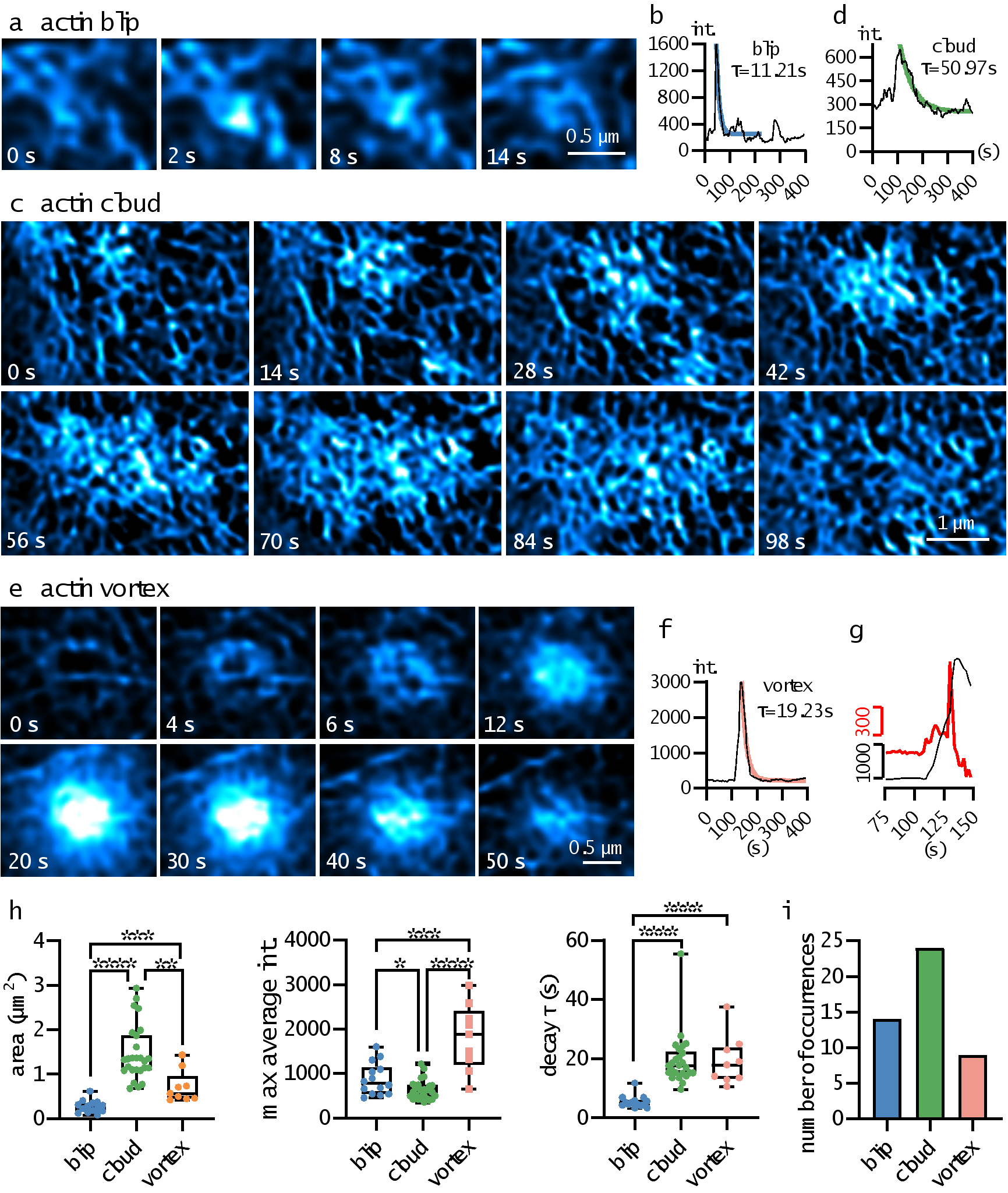
Figure 4. Examples of the distribution of three novel local dynamic processes of actin filaments, along with the corresponding fluorescence over time curve. Additionally, statistical results of parameters such as the distribution area, average fluorescence signal peak, fluorescence decay time constant, and occurrence frequency of the local dynamic processes are provided.
In summary, this paper proposes a background filtering method based on a physical model, which effectively reduces artifacts and ensures signal linearity and structural integrity while improving resolution through sparse deconvolution.
Yanquan Mo, a doctoral student at the College of Future Technology of Peking University, is the first author of this paper. Professor Liangyi Chen from the School of Future Technology of Peking University and Associate Professor JunChao Fan from Chongqing University of Posts and Telecommunications are the corresponding authors of the paper. This research also received assistance from Kunhao Wang of South China Normal University, Liuju Li and Shijia Xing of the School of Future Technology of Peking University. The data processing of this project was supported by the hardware and technical support of the Peking University Unnamed Supercomputer Platform. This project was supported by the National Natural Science Foundation, the Ministry of Science and Technology, and the Joint Center for Life Sciences, among others.
Reference
1.Huang, X. et al. Fast, long-term, super-resolution imaging with Hessian structured illumination microscopy. Nat. Biotechnol.36, 451–459 (2018).
2.Zhao, W. et al. Sparse deconvolution improves the resolution of live-cell super-resolution fluorescence microscopy. Nat. Biotechnol.40, 606–617 (2022).
3.Müller, M., Mönkemöller, V., Hennig, S., Hübner, W. & Huser, T. Open-source image reconstruction of super-resolution structured illumination microscopy data in ImageJ. Nat. Commun.7, 10980 (2016).
4.Wicker, K., Mandula, O., Best, G., Fiolka, R. & Heintzmann, R. Phase optimisation for structured illumination microscopy. Opt. Express21, 2032–2049 (2013).
5.Wen, G. et al. High-fidelity structured illumination microscopy by point-spread-function engineering. Light. Sci. Appl.10, 70 (2021).
6. Mo, Y. et al. Quantitative structured illumination microscopy via a physical model-based background filtering algorithm reveals actin dynamics. Nat Commun14, 3089 (2023).




